4CPS-175 Effectiveness of immunotherapy as a function of age: meta-analysis of the approved combinations in first-line metastatic non-small-cell lung cancer in patients without mutations Article Swipe
 YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1136/ejhpharm-2024-eahp.279
YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1136/ejhpharm-2024-eahp.279
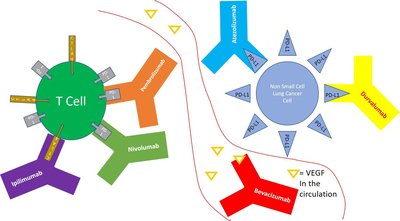
Background and Importance It could be hypothesised that patients older than 65 years old may experience decreased immune function due to the natural aging process, which could lead to a more limited response to immunotherapy compared to those younger than 65 years old. The forest-plot analysis for age-dependent overall survival from the clinical trial of cemiplimab in combination with chemotherapy in locally advanced or metastatic non-small-cell lung cancer (NSCLC), EMPOWER-Lung 3, showed a borderline interaction between the subgroups younger and older than 65 years old, with a p-interaction=0.0895 (own calculation) and HR 0.53 (0.39–0.72), HR 0.81 (0.55–1.18), respectively. Aim and Objectives To verify the consistency of the hypothesis of an age-related effectiveness by a meta-analysis considering all approved immunotherapy combinations in first-line NSCLC. Material and Methods A MEDLINE-PubMed literature search was conducted for phase III randomised clinical trials (RCTs) with similar population and duration of pembrolizumab, atezolizumab ± bevacizumab, nivolumab + ipilimumab, durvalumab + tremelimumab and cemiplimab, in combination with chemotherapy and nivolumab + ipilimumab. A meta-analysis was performed with the MetaSurv calculator. The primary endpoint was overall survival (OS) in patients younger and older than, or equal to, 65 years of age. Age-dependent OS data for immunotherapy combinations versus a common comparator, platinum-based chemotherapy, were compared. Interaction was considered significative if p<0.05 and doubtful if 0.05≤p<0.1. Results A pooled HR of 0.67 (95% CI 0.58–0.76), p<0.000001 was obtained in patients younger than 65 years of age. Heterogeneity among trials estimate values were as follows: Q 14.84, p=0.03812. I2 53% (CI 95% 0–79%). In those older than 65 years old, the combined HR obtained was 0.77 (95% CI 0.70–0.84), p<0.000001. Heterogeneity estimate values were as follows: Q for heterogeneity 0.81 p=0.99733. I2 0% (CI 95% 0–0%). The calculated p-interaction between the combined HRs of the under-65 and over-65 groups was 0.0551, which is considered a doubtful interaction in a subgroup analysis. Conclusion and Relevance A significant benefit for immunotherapy-chemotherapy over chemotherapy alone was shown in both age groups. There is some consistency regarding a greater effectiveness of immunotherapy in patients under 65 years of age, but more data would be needed to confirm this possible difference. References and/or Acknowledgements Conflict of Interest No conflict of interest.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1136/ejhpharm-2024-eahp.279
- https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdf
- OA Status
- bronze
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4393006041
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4393006041Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1136/ejhpharm-2024-eahp.279Digital Object Identifier
- Title
-
4CPS-175 Effectiveness of immunotherapy as a function of age: meta-analysis of the approved combinations in first-line metastatic non-small-cell lung cancer in patients without mutationsWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2024Year of publication
- Publication date
-
2024-03-01Full publication date if available
- Authors
-
A Aguado Paredes, Emilio Jesús Alegre Del ReyList of authors in order
- Landing page
-
https://doi.org/10.1136/ejhpharm-2024-eahp.279Publisher landing page
- PDF URL
-
https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
bronzeOpen access status per OpenAlex
- OA URL
-
https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdfDirect OA link when available
- Concepts
-
Ipilimumab, Medicine, Nivolumab, Atezolizumab, Internal medicine, Oncology, Durvalumab, Pembrolizumab, Lung cancer, Bevacizumab, Meta-analysis, Population, Immunotherapy, Chemotherapy, Cancer, Environmental healthTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4393006041 |
|---|---|
| doi | https://doi.org/10.1136/ejhpharm-2024-eahp.279 |
| ids.doi | https://doi.org/10.1136/ejhpharm-2024-eahp.279 |
| ids.openalex | https://openalex.org/W4393006041 |
| fwci | 0.0 |
| type | article |
| title | 4CPS-175 Effectiveness of immunotherapy as a function of age: meta-analysis of the approved combinations in first-line metastatic non-small-cell lung cancer in patients without mutations |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | A136 |
| biblio.first_page | A135.2 |
| topics[0].id | https://openalex.org/T10158 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9990000128746033 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2730 |
| topics[0].subfield.display_name | Oncology |
| topics[0].display_name | Cancer Immunotherapy and Biomarkers |
| topics[1].id | https://openalex.org/T11067 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9858999848365784 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2730 |
| topics[1].subfield.display_name | Oncology |
| topics[1].display_name | Colorectal Cancer Treatments and Studies |
| topics[2].id | https://openalex.org/T10417 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9822999835014343 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2740 |
| topics[2].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[2].display_name | Lung Cancer Treatments and Mutations |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C2781433595 |
| concepts[0].level | 4 |
| concepts[0].score | 0.8821773529052734 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q2459042 |
| concepts[0].display_name | Ipilimumab |
| concepts[1].id | https://openalex.org/C71924100 |
| concepts[1].level | 0 |
| concepts[1].score | 0.8465301394462585 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[1].display_name | Medicine |
| concepts[2].id | https://openalex.org/C2780030458 |
| concepts[2].level | 4 |
| concepts[2].score | 0.8170089721679688 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q7041828 |
| concepts[2].display_name | Nivolumab |
| concepts[3].id | https://openalex.org/C2775949291 |
| concepts[3].level | 5 |
| concepts[3].score | 0.7851433753967285 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q20707748 |
| concepts[3].display_name | Atezolizumab |
| concepts[4].id | https://openalex.org/C126322002 |
| concepts[4].level | 1 |
| concepts[4].score | 0.678189754486084 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[4].display_name | Internal medicine |
| concepts[5].id | https://openalex.org/C143998085 |
| concepts[5].level | 1 |
| concepts[5].score | 0.6714179515838623 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q162555 |
| concepts[5].display_name | Oncology |
| concepts[6].id | https://openalex.org/C2777742743 |
| concepts[6].level | 5 |
| concepts[6].score | 0.5204082727432251 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q19904005 |
| concepts[6].display_name | Durvalumab |
| concepts[7].id | https://openalex.org/C2780057760 |
| concepts[7].level | 4 |
| concepts[7].score | 0.49143660068511963 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q13896859 |
| concepts[7].display_name | Pembrolizumab |
| concepts[8].id | https://openalex.org/C2776256026 |
| concepts[8].level | 2 |
| concepts[8].score | 0.47096067667007446 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q47912 |
| concepts[8].display_name | Lung cancer |
| concepts[9].id | https://openalex.org/C2777802072 |
| concepts[9].level | 3 |
| concepts[9].score | 0.4213330149650574 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q413299 |
| concepts[9].display_name | Bevacizumab |
| concepts[10].id | https://openalex.org/C95190672 |
| concepts[10].level | 2 |
| concepts[10].score | 0.4183506369590759 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q815382 |
| concepts[10].display_name | Meta-analysis |
| concepts[11].id | https://openalex.org/C2908647359 |
| concepts[11].level | 2 |
| concepts[11].score | 0.4111616611480713 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q2625603 |
| concepts[11].display_name | Population |
| concepts[12].id | https://openalex.org/C2777701055 |
| concepts[12].level | 3 |
| concepts[12].score | 0.4059470295906067 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q1427096 |
| concepts[12].display_name | Immunotherapy |
| concepts[13].id | https://openalex.org/C2776694085 |
| concepts[13].level | 2 |
| concepts[13].score | 0.33248668909072876 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q974135 |
| concepts[13].display_name | Chemotherapy |
| concepts[14].id | https://openalex.org/C121608353 |
| concepts[14].level | 2 |
| concepts[14].score | 0.32731103897094727 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[14].display_name | Cancer |
| concepts[15].id | https://openalex.org/C99454951 |
| concepts[15].level | 1 |
| concepts[15].score | 0.0 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q932068 |
| concepts[15].display_name | Environmental health |
| keywords[0].id | https://openalex.org/keywords/ipilimumab |
| keywords[0].score | 0.8821773529052734 |
| keywords[0].display_name | Ipilimumab |
| keywords[1].id | https://openalex.org/keywords/medicine |
| keywords[1].score | 0.8465301394462585 |
| keywords[1].display_name | Medicine |
| keywords[2].id | https://openalex.org/keywords/nivolumab |
| keywords[2].score | 0.8170089721679688 |
| keywords[2].display_name | Nivolumab |
| keywords[3].id | https://openalex.org/keywords/atezolizumab |
| keywords[3].score | 0.7851433753967285 |
| keywords[3].display_name | Atezolizumab |
| keywords[4].id | https://openalex.org/keywords/internal-medicine |
| keywords[4].score | 0.678189754486084 |
| keywords[4].display_name | Internal medicine |
| keywords[5].id | https://openalex.org/keywords/oncology |
| keywords[5].score | 0.6714179515838623 |
| keywords[5].display_name | Oncology |
| keywords[6].id | https://openalex.org/keywords/durvalumab |
| keywords[6].score | 0.5204082727432251 |
| keywords[6].display_name | Durvalumab |
| keywords[7].id | https://openalex.org/keywords/pembrolizumab |
| keywords[7].score | 0.49143660068511963 |
| keywords[7].display_name | Pembrolizumab |
| keywords[8].id | https://openalex.org/keywords/lung-cancer |
| keywords[8].score | 0.47096067667007446 |
| keywords[8].display_name | Lung cancer |
| keywords[9].id | https://openalex.org/keywords/bevacizumab |
| keywords[9].score | 0.4213330149650574 |
| keywords[9].display_name | Bevacizumab |
| keywords[10].id | https://openalex.org/keywords/meta-analysis |
| keywords[10].score | 0.4183506369590759 |
| keywords[10].display_name | Meta-analysis |
| keywords[11].id | https://openalex.org/keywords/population |
| keywords[11].score | 0.4111616611480713 |
| keywords[11].display_name | Population |
| keywords[12].id | https://openalex.org/keywords/immunotherapy |
| keywords[12].score | 0.4059470295906067 |
| keywords[12].display_name | Immunotherapy |
| keywords[13].id | https://openalex.org/keywords/chemotherapy |
| keywords[13].score | 0.33248668909072876 |
| keywords[13].display_name | Chemotherapy |
| keywords[14].id | https://openalex.org/keywords/cancer |
| keywords[14].score | 0.32731103897094727 |
| keywords[14].display_name | Cancer |
| language | en |
| locations[0].id | doi:10.1136/ejhpharm-2024-eahp.279 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S4363605422 |
| locations[0].source.issn | |
| locations[0].source.type | conference |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | |
| locations[0].source.is_core | False |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Section 4: Clinical pharmacy services |
| locations[0].source.host_organization | |
| locations[0].source.host_organization_name | |
| locations[0].license | |
| locations[0].pdf_url | https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | proceedings-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Section 4: Clinical pharmacy services |
| locations[0].landing_page_url | https://doi.org/10.1136/ejhpharm-2024-eahp.279 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A5035265085 |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | A Aguado Paredes |
| authorships[0].countries | ES |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I4210090436 |
| authorships[0].affiliations[0].raw_affiliation_string | Hospital Universitario Virgen Macarena, Clinical Pharmacy, Sevilla, Spain |
| authorships[0].institutions[0].id | https://openalex.org/I4210090436 |
| authorships[0].institutions[0].ror | https://ror.org/016p83279 |
| authorships[0].institutions[0].type | healthcare |
| authorships[0].institutions[0].lineage | https://openalex.org/I4210090436 |
| authorships[0].institutions[0].country_code | ES |
| authorships[0].institutions[0].display_name | Hospital Universitario Virgen Macarena |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | A Aguado Paredes |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Hospital Universitario Virgen Macarena, Clinical Pharmacy, Sevilla, Spain |
| authorships[1].author.id | https://openalex.org/A5080654981 |
| authorships[1].author.orcid | https://orcid.org/0000-0003-0041-2024 |
| authorships[1].author.display_name | Emilio Jesús Alegre Del Rey |
| authorships[1].countries | ES |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I4210137875 |
| authorships[1].affiliations[0].raw_affiliation_string | Hospital Universitario Puerto Real, Clinical Pharmacy, Cádiz, Spain |
| authorships[1].institutions[0].id | https://openalex.org/I4210137875 |
| authorships[1].institutions[0].ror | https://ror.org/04fbqvq73 |
| authorships[1].institutions[0].type | healthcare |
| authorships[1].institutions[0].lineage | https://openalex.org/I158283456, https://openalex.org/I4210137875 |
| authorships[1].institutions[0].country_code | ES |
| authorships[1].institutions[0].display_name | Hospital Universitario Puerto Real |
| authorships[1].author_position | last |
| authorships[1].raw_author_name | EJ Alegre Del Rey |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Hospital Universitario Puerto Real, Clinical Pharmacy, Cádiz, Spain |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdf |
| open_access.oa_status | bronze |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | 4CPS-175 Effectiveness of immunotherapy as a function of age: meta-analysis of the approved combinations in first-line metastatic non-small-cell lung cancer in patients without mutations |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10158 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9990000128746033 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2730 |
| primary_topic.subfield.display_name | Oncology |
| primary_topic.display_name | Cancer Immunotherapy and Biomarkers |
| related_works | https://openalex.org/W4400043472, https://openalex.org/W4386284132, https://openalex.org/W3210901691, https://openalex.org/W4313890856, https://openalex.org/W2766929851, https://openalex.org/W3210130056, https://openalex.org/W4393305029, https://openalex.org/W3017309209, https://openalex.org/W4206430965, https://openalex.org/W2969038597 |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | doi:10.1136/ejhpharm-2024-eahp.279 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S4363605422 |
| best_oa_location.source.issn | |
| best_oa_location.source.type | conference |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | |
| best_oa_location.source.is_core | False |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | Section 4: Clinical pharmacy services |
| best_oa_location.source.host_organization | |
| best_oa_location.source.host_organization_name | |
| best_oa_location.license | |
| best_oa_location.pdf_url | https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | proceedings-article |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Section 4: Clinical pharmacy services |
| best_oa_location.landing_page_url | https://doi.org/10.1136/ejhpharm-2024-eahp.279 |
| primary_location.id | doi:10.1136/ejhpharm-2024-eahp.279 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S4363605422 |
| primary_location.source.issn | |
| primary_location.source.type | conference |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | |
| primary_location.source.is_core | False |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Section 4: Clinical pharmacy services |
| primary_location.source.host_organization | |
| primary_location.source.host_organization_name | |
| primary_location.license | |
| primary_location.pdf_url | https://ejhp.bmj.com/content/ejhpharm/31/Suppl_1/A135.2.full.pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | proceedings-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Section 4: Clinical pharmacy services |
| primary_location.landing_page_url | https://doi.org/10.1136/ejhpharm-2024-eahp.279 |
| publication_date | 2024-03-01 |
| publication_year | 2024 |
| referenced_works_count | 0 |
| abstract_inverted_index.+ | 150, 153, 163 |
| abstract_inverted_index.A | 126, 165, 218, 314 |
| abstract_inverted_index.Q | 245, 276 |
| abstract_inverted_index.a | 29, 72, 86, 113, 200, 304, 308, 333 |
| abstract_inverted_index.0% | 282 |
| abstract_inverted_index.3, | 70 |
| abstract_inverted_index.65 | 11, 40, 82, 189, 233, 257, 341 |
| abstract_inverted_index.CI | 224, 267 |
| abstract_inverted_index.HR | 91, 94, 220, 262 |
| abstract_inverted_index.I2 | 248, 281 |
| abstract_inverted_index.In | 253 |
| abstract_inverted_index.It | 3 |
| abstract_inverted_index.No | 362 |
| abstract_inverted_index.OS | 194 |
| abstract_inverted_index.To | 101 |
| abstract_inverted_index.an | 109 |
| abstract_inverted_index.as | 243, 274 |
| abstract_inverted_index.be | 5, 349 |
| abstract_inverted_index.by | 112 |
| abstract_inverted_index.if | 211, 215 |
| abstract_inverted_index.in | 56, 60, 120, 157, 180, 229, 307, 324, 338 |
| abstract_inverted_index.is | 302, 329 |
| abstract_inverted_index.of | 54, 105, 108, 144, 191, 221, 235, 293, 336, 343, 360, 364 |
| abstract_inverted_index.or | 63, 186 |
| abstract_inverted_index.to | 20, 28, 33, 36, 351 |
| abstract_inverted_index.± | 147 |
| abstract_inverted_index.(CI | 250, 283 |
| abstract_inverted_index.53% | 249 |
| abstract_inverted_index.95% | 251, 284 |
| abstract_inverted_index.HRs | 292 |
| abstract_inverted_index.III | 134 |
| abstract_inverted_index.The | 43, 173, 286 |
| abstract_inverted_index.age | 326 |
| abstract_inverted_index.all | 116 |
| abstract_inverted_index.and | 1, 79, 90, 99, 124, 142, 155, 161, 183, 213, 296, 312 |
| abstract_inverted_index.but | 345 |
| abstract_inverted_index.due | 19 |
| abstract_inverted_index.for | 46, 132, 196, 277, 317 |
| abstract_inverted_index.may | 14 |
| abstract_inverted_index.old | 13 |
| abstract_inverted_index.the | 21, 51, 76, 103, 106, 170, 260, 290, 294 |
| abstract_inverted_index.to, | 188 |
| abstract_inverted_index.was | 130, 167, 176, 208, 227, 264, 299, 322 |
| abstract_inverted_index.(95% | 223, 266 |
| abstract_inverted_index.(OS) | 179 |
| abstract_inverted_index.(own | 88 |
| abstract_inverted_index.0.53 | 92 |
| abstract_inverted_index.0.67 | 222 |
| abstract_inverted_index.0.77 | 265 |
| abstract_inverted_index.0.81 | 95, 279 |
| abstract_inverted_index.age, | 344 |
| abstract_inverted_index.age. | 192, 236 |
| abstract_inverted_index.both | 325 |
| abstract_inverted_index.data | 195, 347 |
| abstract_inverted_index.from | 50 |
| abstract_inverted_index.lead | 27 |
| abstract_inverted_index.lung | 66 |
| abstract_inverted_index.more | 30, 346 |
| abstract_inverted_index.old, | 84, 259 |
| abstract_inverted_index.old. | 42 |
| abstract_inverted_index.over | 319 |
| abstract_inverted_index.some | 330 |
| abstract_inverted_index.than | 10, 39, 81, 232, 256 |
| abstract_inverted_index.that | 7 |
| abstract_inverted_index.this | 353 |
| abstract_inverted_index.were | 205, 242, 273 |
| abstract_inverted_index.with | 58, 85, 139, 159, 169 |
| abstract_inverted_index.There | 328 |
| abstract_inverted_index.aging | 23 |
| abstract_inverted_index.alone | 321 |
| abstract_inverted_index.among | 238 |
| abstract_inverted_index.could | 4, 26 |
| abstract_inverted_index.equal | 187 |
| abstract_inverted_index.older | 9, 80, 184, 255 |
| abstract_inverted_index.phase | 133 |
| abstract_inverted_index.shown | 323 |
| abstract_inverted_index.than, | 185 |
| abstract_inverted_index.those | 37, 254 |
| abstract_inverted_index.trial | 53 |
| abstract_inverted_index.under | 340 |
| abstract_inverted_index.which | 25, 301 |
| abstract_inverted_index.would | 348 |
| abstract_inverted_index.years | 12, 41, 83, 190, 234, 258, 342 |
| abstract_inverted_index.(RCTs) | 138 |
| abstract_inverted_index.14.84, | 246 |
| abstract_inverted_index.NSCLC. | 122 |
| abstract_inverted_index.and/or | 357 |
| abstract_inverted_index.cancer | 67 |
| abstract_inverted_index.common | 201 |
| abstract_inverted_index.groups | 298 |
| abstract_inverted_index.immune | 17 |
| abstract_inverted_index.needed | 350 |
| abstract_inverted_index.pooled | 219 |
| abstract_inverted_index.search | 129 |
| abstract_inverted_index.showed | 71 |
| abstract_inverted_index.trials | 137, 239 |
| abstract_inverted_index.values | 241, 272 |
| abstract_inverted_index.verify | 102 |
| abstract_inverted_index.versus | 199 |
| abstract_inverted_index.0.0551, | 300 |
| abstract_inverted_index.<h3>Aim | 98 |
| abstract_inverted_index.benefit | 316 |
| abstract_inverted_index.between | 75, 289 |
| abstract_inverted_index.confirm | 352 |
| abstract_inverted_index.greater | 334 |
| abstract_inverted_index.groups. | 327 |
| abstract_inverted_index.limited | 31 |
| abstract_inverted_index.locally | 61 |
| abstract_inverted_index.natural | 22 |
| abstract_inverted_index.over-65 | 297 |
| abstract_inverted_index.overall | 48, 177 |
| abstract_inverted_index.primary | 174 |
| abstract_inverted_index.similar | 140 |
| abstract_inverted_index.younger | 38, 78, 182, 231 |
| abstract_inverted_index.(NSCLC), | 68 |
| abstract_inverted_index.0–0%). | 285 |
| abstract_inverted_index.MetaSurv | 171 |
| abstract_inverted_index.advanced | 62 |
| abstract_inverted_index.analysis | 45 |
| abstract_inverted_index.approved | 117 |
| abstract_inverted_index.clinical | 52, 136 |
| abstract_inverted_index.combined | 261, 291 |
| abstract_inverted_index.compared | 35 |
| abstract_inverted_index.conflict | 363 |
| abstract_inverted_index.doubtful | 214, 305 |
| abstract_inverted_index.duration | 143 |
| abstract_inverted_index.endpoint | 175 |
| abstract_inverted_index.estimate | 240, 271 |
| abstract_inverted_index.follows: | 244, 275 |
| abstract_inverted_index.function | 18 |
| abstract_inverted_index.obtained | 228, 263 |
| abstract_inverted_index.patients | 8, 181, 230, 339 |
| abstract_inverted_index.possible | 354 |
| abstract_inverted_index.process, | 24 |
| abstract_inverted_index.response | 32 |
| abstract_inverted_index.subgroup | 309 |
| abstract_inverted_index.survival | 49, 178 |
| abstract_inverted_index.under-65 | 295 |
| abstract_inverted_index.0–79%). | 252 |
| abstract_inverted_index.analysis. | 310 |
| abstract_inverted_index.compared. | 206 |
| abstract_inverted_index.conducted | 131 |
| abstract_inverted_index.decreased | 16 |
| abstract_inverted_index.interest. | 365 |
| abstract_inverted_index.nivolumab | 149, 162 |
| abstract_inverted_index.p<0.05 | 212 |
| abstract_inverted_index.performed | 168 |
| abstract_inverted_index.regarding | 332 |
| abstract_inverted_index.subgroups | 77 |
| abstract_inverted_index.borderline | 73 |
| abstract_inverted_index.calculated | 287 |
| abstract_inverted_index.cemiplimab | 55 |
| abstract_inverted_index.considered | 209, 303 |
| abstract_inverted_index.durvalumab | 152 |
| abstract_inverted_index.experience | 15 |
| abstract_inverted_index.first-line | 121 |
| abstract_inverted_index.hypothesis | 107 |
| abstract_inverted_index.literature | 128 |
| abstract_inverted_index.metastatic | 64 |
| abstract_inverted_index.p=0.03812. | 247 |
| abstract_inverted_index.p=0.99733. | 280 |
| abstract_inverted_index.population | 141 |
| abstract_inverted_index.randomised | 135 |
| abstract_inverted_index.Interaction | 207 |
| abstract_inverted_index.age-related | 110 |
| abstract_inverted_index.calculator. | 172 |
| abstract_inverted_index.cemiplimab, | 156 |
| abstract_inverted_index.combination | 57, 158 |
| abstract_inverted_index.comparator, | 202 |
| abstract_inverted_index.considering | 115 |
| abstract_inverted_index.consistency | 104, 331 |
| abstract_inverted_index.difference. | 355 |
| abstract_inverted_index.forest-plot | 44 |
| abstract_inverted_index.interaction | 74, 306 |
| abstract_inverted_index.ipilimumab, | 151 |
| abstract_inverted_index.ipilimumab. | 164 |
| abstract_inverted_index.significant | 315 |
| abstract_inverted_index.<h3>Conflict | 359 |
| abstract_inverted_index.<h3>Material | 123 |
| abstract_inverted_index.EMPOWER-Lung | 69 |
| abstract_inverted_index.Methods</h3> | 125 |
| abstract_inverted_index.atezolizumab | 146 |
| abstract_inverted_index.bevacizumab, | 148 |
| abstract_inverted_index.calculation) | 89 |
| abstract_inverted_index.chemotherapy | 59, 160, 320 |
| abstract_inverted_index.combinations | 119, 198 |
| abstract_inverted_index.hypothesised | 6 |
| abstract_inverted_index.tremelimumab | 154 |
| abstract_inverted_index.0.58–0.76), | 225 |
| abstract_inverted_index.0.70–0.84), | 268 |
| abstract_inverted_index.Age-dependent | 193 |
| abstract_inverted_index.Heterogeneity | 237, 270 |
| abstract_inverted_index.Interest</h3> | 361 |
| abstract_inverted_index.age-dependent | 47 |
| abstract_inverted_index.chemotherapy, | 204 |
| abstract_inverted_index.effectiveness | 111, 335 |
| abstract_inverted_index.heterogeneity | 278 |
| abstract_inverted_index.immunotherapy | 34, 118, 197, 337 |
| abstract_inverted_index.meta-analysis | 114, 166 |
| abstract_inverted_index.p<0.000001 | 226 |
| abstract_inverted_index.p-interaction | 288 |
| abstract_inverted_index.respectively. | 97 |
| abstract_inverted_index.significative | 210 |
| abstract_inverted_index.(0.39–0.72), | 93 |
| abstract_inverted_index.(0.55–1.18), | 96 |
| abstract_inverted_index.<h3>Background | 0 |
| abstract_inverted_index.<h3>Conclusion | 311 |
| abstract_inverted_index.<h3>References | 356 |
| abstract_inverted_index.MEDLINE-PubMed | 127 |
| abstract_inverted_index.Relevance</h3> | 313 |
| abstract_inverted_index.non-small-cell | 65 |
| abstract_inverted_index.p<0.000001. | 269 |
| abstract_inverted_index.pembrolizumab, | 145 |
| abstract_inverted_index.platinum-based | 203 |
| abstract_inverted_index.Importance</h3> | 2 |
| abstract_inverted_index.Objectives</h3> | 100 |
| abstract_inverted_index.0.05≤p<0.1. | 216 |
| abstract_inverted_index.<h3>Results</h3> | 217 |
| abstract_inverted_index.p-interaction=0.0895 | 87 |
| abstract_inverted_index.Acknowledgements</h3> | 358 |
| abstract_inverted_index.immunotherapy-chemotherapy | 318 |
| cited_by_percentile_year | |
| countries_distinct_count | 1 |
| institutions_distinct_count | 2 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.75 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.15789474 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |