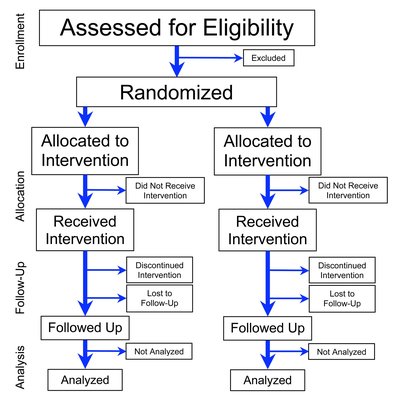
A 2 x 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial: a protocol for the CLEAR clinical trial Article Swipe
 YOU?
·
· 2019
· Open Access
·
YOU?
·
· 2019
· Open Access
·
Background: Current guidelines for the management of bronchiectasis (BE) highlight the lack of evidence to recommend mucoactive agents, such as hypertonic saline (HTS) and carbocisteine, to aid sputum removal as part of standard care. We hypothesise that mucoactive agents (HTS or carbocisteine, or a combination) are effective in reducing exacerbations over a 52-week period, compared to usual care. Methods: This is a 52-week, 2 × 2 factorial, randomized, open-label trial to determine the clinical effectiveness and cost effectiveness of HTS 6% and carbocisteine for airway clearance versus usual care-the Clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care (CLEAR) trial. Patients will be randomised to (1) standard care and twice-daily nebulised HTS (6%), (2) standard care and carbocisteine (750 mg three times per day until visit 3, reducing to 750 mg twice per day), (3) standard care and combination of twice-daily nebulised HTS and carbocisteine, or (4) standard care. The primary outcome is the mean number of exacerbations over 52 weeks. Key inclusion criteria are as follows: Adults with a diagnosis of BE on computed tomography, BE as the primary respiratory diagnosis, and two or more pulmonary exacerbations in the last year requiring antibiotics and production of daily sputum. Discussion: This trial's pragmatic research design avoids the significant costs associated with double-blind trials whilst optimising rigour in other areas of trial delivery. The CLEAR trial will provide evidence as to whether HTS, carbocisteine or both are effective and cost effective for patients with BE. Trial registration: EudraCT number: 2017-000664-14 (first entered in the database on 20 October 2017). ISRCTN.com, ISRCTN89040295. Registered on 6 July/2018. Funder: National Institute for Health Research, Health Technology Assessment Programme (15/100/01). Sponsor: Belfast Health and Social Care Trust. Ethics Reference Number: 17/NE/0339. Protocol version: V3.0 Final_14052018.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://orcid.org/0000-0002-5367-9841>,
- http://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdf
- OA Status
- green
- Related Works
- 20
- OpenAlex ID
- https://openalex.org/W3037080114
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W3037080114Canonical identifier for this work in OpenAlex
- Title
-
A 2 x 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial: a protocol for the CLEAR clinical trialWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2019Year of publication
- Publication date
-
2019-12-19Full publication date if available
- Authors
-
Judy Bradley, Rohan Anand, Brenda O’Neill, Kathryn M. Ferguson, Mike Clarke, Mary Carroll, James D. Chalmers, Anthony De Soyza, J. Duckers, Adam T. Hill, Michael R. Loebinger, Fiona Copeland, Evie Gardner, Christina Campbell, Ashley Agus, Alistair McGuire, Roisin Boyle, Fionnuala McKinney, Naomi Dickson, Daniel F. McAuley, J.S. ElbornList of authors in order
- Landing page
-
https://orcid.org/0000-0002-5367-9841>,Publisher landing page
- PDF URL
-
https://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
greenOpen access status per OpenAlex
- OA URL
-
https://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdfDirect OA link when available
- Concepts
-
Hypertonic saline, Medicine, Sputum, Randomized controlled trial, Clinical trial, Intensive care medicine, Cost effectiveness, Anesthesia, Internal medicine, Tuberculosis, Pathology, Risk analysis (engineering)Top concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- Related works (count)
-
20Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W3037080114 |
|---|---|
| doi | |
| ids.mag | 3037080114 |
| ids.openalex | https://openalex.org/W3037080114 |
| fwci | 0.0 |
| type | article |
| title | A 2 x 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial: a protocol for the CLEAR clinical trial |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | |
| biblio.first_page | |
| topics[0].id | https://openalex.org/T10665 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9980000257492065 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2740 |
| topics[0].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[0].display_name | Cystic Fibrosis Research Advances |
| topics[1].id | https://openalex.org/T10143 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9976999759674072 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2740 |
| topics[1].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[1].display_name | Chronic Obstructive Pulmonary Disease (COPD) Research |
| topics[2].id | https://openalex.org/T10549 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9976000189781189 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2740 |
| topics[2].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[2].display_name | Neonatal Respiratory Health Research |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C3017567848 |
| concepts[0].level | 2 |
| concepts[0].score | 0.8693234920501709 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q275792 |
| concepts[0].display_name | Hypertonic saline |
| concepts[1].id | https://openalex.org/C71924100 |
| concepts[1].level | 0 |
| concepts[1].score | 0.7802762389183044 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[1].display_name | Medicine |
| concepts[2].id | https://openalex.org/C2776301714 |
| concepts[2].level | 3 |
| concepts[2].score | 0.6691657304763794 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q259346 |
| concepts[2].display_name | Sputum |
| concepts[3].id | https://openalex.org/C168563851 |
| concepts[3].level | 2 |
| concepts[3].score | 0.5638813376426697 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[3].display_name | Randomized controlled trial |
| concepts[4].id | https://openalex.org/C535046627 |
| concepts[4].level | 2 |
| concepts[4].score | 0.4985697269439697 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[4].display_name | Clinical trial |
| concepts[5].id | https://openalex.org/C177713679 |
| concepts[5].level | 1 |
| concepts[5].score | 0.47659891843795776 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q679690 |
| concepts[5].display_name | Intensive care medicine |
| concepts[6].id | https://openalex.org/C3019080777 |
| concepts[6].level | 2 |
| concepts[6].score | 0.4665975570678711 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q1754768 |
| concepts[6].display_name | Cost effectiveness |
| concepts[7].id | https://openalex.org/C42219234 |
| concepts[7].level | 1 |
| concepts[7].score | 0.3312547504901886 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q131130 |
| concepts[7].display_name | Anesthesia |
| concepts[8].id | https://openalex.org/C126322002 |
| concepts[8].level | 1 |
| concepts[8].score | 0.2537640333175659 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[8].display_name | Internal medicine |
| concepts[9].id | https://openalex.org/C2781069245 |
| concepts[9].level | 2 |
| concepts[9].score | 0.07034507393836975 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q12204 |
| concepts[9].display_name | Tuberculosis |
| concepts[10].id | https://openalex.org/C142724271 |
| concepts[10].level | 1 |
| concepts[10].score | 0.06227418780326843 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q7208 |
| concepts[10].display_name | Pathology |
| concepts[11].id | https://openalex.org/C112930515 |
| concepts[11].level | 1 |
| concepts[11].score | 0.0 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q4389547 |
| concepts[11].display_name | Risk analysis (engineering) |
| keywords[0].id | https://openalex.org/keywords/hypertonic-saline |
| keywords[0].score | 0.8693234920501709 |
| keywords[0].display_name | Hypertonic saline |
| keywords[1].id | https://openalex.org/keywords/medicine |
| keywords[1].score | 0.7802762389183044 |
| keywords[1].display_name | Medicine |
| keywords[2].id | https://openalex.org/keywords/sputum |
| keywords[2].score | 0.6691657304763794 |
| keywords[2].display_name | Sputum |
| keywords[3].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[3].score | 0.5638813376426697 |
| keywords[3].display_name | Randomized controlled trial |
| keywords[4].id | https://openalex.org/keywords/clinical-trial |
| keywords[4].score | 0.4985697269439697 |
| keywords[4].display_name | Clinical trial |
| keywords[5].id | https://openalex.org/keywords/intensive-care-medicine |
| keywords[5].score | 0.47659891843795776 |
| keywords[5].display_name | Intensive care medicine |
| keywords[6].id | https://openalex.org/keywords/cost-effectiveness |
| keywords[6].score | 0.4665975570678711 |
| keywords[6].display_name | Cost effectiveness |
| keywords[7].id | https://openalex.org/keywords/anesthesia |
| keywords[7].score | 0.3312547504901886 |
| keywords[7].display_name | Anesthesia |
| keywords[8].id | https://openalex.org/keywords/internal-medicine |
| keywords[8].score | 0.2537640333175659 |
| keywords[8].display_name | Internal medicine |
| keywords[9].id | https://openalex.org/keywords/tuberculosis |
| keywords[9].score | 0.07034507393836975 |
| keywords[9].display_name | Tuberculosis |
| keywords[10].id | https://openalex.org/keywords/pathology |
| keywords[10].score | 0.06227418780326843 |
| keywords[10].display_name | Pathology |
| language | en |
| locations[0].id | pmh:oai:eprints.lse.ac.uk:103017 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S4306401593 |
| locations[0].source.issn | |
| locations[0].source.type | repository |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | |
| locations[0].source.is_core | False |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | London School of Economics and Political Science Research Online (London School of Economics and Political Science) |
| locations[0].source.host_organization | https://openalex.org/I909854389 |
| locations[0].source.host_organization_name | London School of Economics and Political Science |
| locations[0].source.host_organization_lineage | https://openalex.org/I909854389 |
| locations[0].license | other-oa |
| locations[0].pdf_url | http://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdf |
| locations[0].version | acceptedVersion |
| locations[0].raw_type | PeerReviewed |
| locations[0].license_id | https://openalex.org/licenses/other-oa |
| locations[0].is_accepted | True |
| locations[0].is_published | False |
| locations[0].raw_source_name | |
| locations[0].landing_page_url | https://orcid.org/0000-0002-5367-9841>, |
| locations[1].id | mag:3037080114 |
| locations[1].is_oa | False |
| locations[1].source | |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | False |
| locations[1].is_published | |
| locations[1].raw_source_name | |
| locations[1].landing_page_url | http://eprints.lse.ac.uk/103017/ |
| authorships[0].author.id | https://openalex.org/A5020601451 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-7423-135X |
| authorships[0].author.display_name | Judy Bradley |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Judy Martina Bradley |
| authorships[0].is_corresponding | False |
| authorships[1].author.id | https://openalex.org/A5004507036 |
| authorships[1].author.orcid | https://orcid.org/0000-0002-1957-5336 |
| authorships[1].author.display_name | Rohan Anand |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Rohan Anand |
| authorships[1].is_corresponding | False |
| authorships[2].author.id | https://openalex.org/A5082960100 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-6471-1413 |
| authorships[2].author.display_name | Brenda O’Neill |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Brenda O'Neill |
| authorships[2].is_corresponding | False |
| authorships[3].author.id | https://openalex.org/A5016119932 |
| authorships[3].author.orcid | https://orcid.org/0000-0002-7671-5403 |
| authorships[3].author.display_name | Kathryn M. Ferguson |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Kathryn Ferguson |
| authorships[3].is_corresponding | False |
| authorships[4].author.id | https://openalex.org/A5068789733 |
| authorships[4].author.orcid | https://orcid.org/0000-0002-2926-7257 |
| authorships[4].author.display_name | Mike Clarke |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Mike Clarke |
| authorships[4].is_corresponding | False |
| authorships[5].author.id | https://openalex.org/A5061029083 |
| authorships[5].author.orcid | https://orcid.org/0000-0001-5396-221X |
| authorships[5].author.display_name | Mary Carroll |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Mary Carroll |
| authorships[5].is_corresponding | False |
| authorships[6].author.id | https://openalex.org/A5056681502 |
| authorships[6].author.orcid | https://orcid.org/0000-0001-5514-7868 |
| authorships[6].author.display_name | James D. Chalmers |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | James Chalmers |
| authorships[6].is_corresponding | False |
| authorships[7].author.id | https://openalex.org/A5070372650 |
| authorships[7].author.orcid | https://orcid.org/0000-0002-8566-0344 |
| authorships[7].author.display_name | Anthony De Soyza |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Anthony De Soyza |
| authorships[7].is_corresponding | False |
| authorships[8].author.id | https://openalex.org/A5082181496 |
| authorships[8].author.orcid | https://orcid.org/0000-0003-3004-279X |
| authorships[8].author.display_name | J. Duckers |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Jamie Duckers |
| authorships[8].is_corresponding | False |
| authorships[9].author.id | https://openalex.org/A5000252949 |
| authorships[9].author.orcid | https://orcid.org/0000-0002-1789-9257 |
| authorships[9].author.display_name | Adam T. Hill |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Adam T. Hill |
| authorships[9].is_corresponding | False |
| authorships[10].author.id | https://openalex.org/A5034835364 |
| authorships[10].author.orcid | https://orcid.org/0000-0002-5965-9913 |
| authorships[10].author.display_name | Michael R. Loebinger |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Michael R. Loebinger |
| authorships[10].is_corresponding | False |
| authorships[11].author.id | https://openalex.org/A5086402495 |
| authorships[11].author.orcid | |
| authorships[11].author.display_name | Fiona Copeland |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Fiona Copeland |
| authorships[11].is_corresponding | False |
| authorships[12].author.id | https://openalex.org/A5062366073 |
| authorships[12].author.orcid | https://orcid.org/0000-0001-8487-4415 |
| authorships[12].author.display_name | Evie Gardner |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | Evie Gardner |
| authorships[12].is_corresponding | False |
| authorships[13].author.id | https://openalex.org/A5045273192 |
| authorships[13].author.orcid | https://orcid.org/0000-0002-8446-523X |
| authorships[13].author.display_name | Christina Campbell |
| authorships[13].author_position | middle |
| authorships[13].raw_author_name | Christina Campbell |
| authorships[13].is_corresponding | False |
| authorships[14].author.id | https://openalex.org/A5090239373 |
| authorships[14].author.orcid | https://orcid.org/0000-0001-9839-6282 |
| authorships[14].author.display_name | Ashley Agus |
| authorships[14].author_position | middle |
| authorships[14].raw_author_name | Ashley Agus |
| authorships[14].is_corresponding | False |
| authorships[15].author.id | https://openalex.org/A5024552865 |
| authorships[15].author.orcid | https://orcid.org/0000-0002-5367-9841 |
| authorships[15].author.display_name | Alistair McGuire |
| authorships[15].author_position | middle |
| authorships[15].raw_author_name | Alistair McGuire |
| authorships[15].is_corresponding | False |
| authorships[16].author.id | https://openalex.org/A5052118474 |
| authorships[16].author.orcid | https://orcid.org/0000-0002-1189-5722 |
| authorships[16].author.display_name | Roisin Boyle |
| authorships[16].author_position | middle |
| authorships[16].raw_author_name | Roisin Boyle |
| authorships[16].is_corresponding | False |
| authorships[17].author.id | https://openalex.org/A5073009982 |
| authorships[17].author.orcid | |
| authorships[17].author.display_name | Fionnuala McKinney |
| authorships[17].author_position | middle |
| authorships[17].raw_author_name | Fionnuala McKinney |
| authorships[17].is_corresponding | False |
| authorships[18].author.id | https://openalex.org/A5027367120 |
| authorships[18].author.orcid | |
| authorships[18].author.display_name | Naomi Dickson |
| authorships[18].author_position | middle |
| authorships[18].raw_author_name | Naomi Dickson |
| authorships[18].is_corresponding | False |
| authorships[19].author.id | https://openalex.org/A5041103166 |
| authorships[19].author.orcid | https://orcid.org/0000-0002-3283-1947 |
| authorships[19].author.display_name | Daniel F. McAuley |
| authorships[19].author_position | middle |
| authorships[19].raw_author_name | Danny F. McAuley |
| authorships[19].is_corresponding | False |
| authorships[20].author.id | https://openalex.org/A5039017673 |
| authorships[20].author.orcid | https://orcid.org/0000-0002-2323-442X |
| authorships[20].author.display_name | J.S. Elborn |
| authorships[20].author_position | last |
| authorships[20].raw_author_name | Stuart Elborn |
| authorships[20].is_corresponding | False |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | http://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdf |
| open_access.oa_status | green |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | A 2 x 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial: a protocol for the CLEAR clinical trial |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T04:12:42.849631 |
| primary_topic.id | https://openalex.org/T10665 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9980000257492065 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2740 |
| primary_topic.subfield.display_name | Pulmonary and Respiratory Medicine |
| primary_topic.display_name | Cystic Fibrosis Research Advances |
| related_works | https://openalex.org/W2995562313, https://openalex.org/W3098462818, https://openalex.org/W2964590601, https://openalex.org/W3126066324, https://openalex.org/W3194494324, https://openalex.org/W3012090411, https://openalex.org/W3017098054, https://openalex.org/W2789901231, https://openalex.org/W3036195002, https://openalex.org/W3114011719, https://openalex.org/W3200839048, https://openalex.org/W2341479106, https://openalex.org/W3120201145, https://openalex.org/W3093956495, https://openalex.org/W3170964438, https://openalex.org/W2811422965, https://openalex.org/W2751605999, https://openalex.org/W3047843067, https://openalex.org/W3038287640, https://openalex.org/W2930676447 |
| cited_by_count | 0 |
| locations_count | 2 |
| best_oa_location.id | pmh:oai:eprints.lse.ac.uk:103017 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S4306401593 |
| best_oa_location.source.issn | |
| best_oa_location.source.type | repository |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | |
| best_oa_location.source.is_core | False |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | London School of Economics and Political Science Research Online (London School of Economics and Political Science) |
| best_oa_location.source.host_organization | https://openalex.org/I909854389 |
| best_oa_location.source.host_organization_name | London School of Economics and Political Science |
| best_oa_location.source.host_organization_lineage | https://openalex.org/I909854389 |
| best_oa_location.license | other-oa |
| best_oa_location.pdf_url | http://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdf |
| best_oa_location.version | acceptedVersion |
| best_oa_location.raw_type | PeerReviewed |
| best_oa_location.license_id | https://openalex.org/licenses/other-oa |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | |
| best_oa_location.landing_page_url | https://orcid.org/0000-0002-5367-9841>, |
| primary_location.id | pmh:oai:eprints.lse.ac.uk:103017 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S4306401593 |
| primary_location.source.issn | |
| primary_location.source.type | repository |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | |
| primary_location.source.is_core | False |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | London School of Economics and Political Science Research Online (London School of Economics and Political Science) |
| primary_location.source.host_organization | https://openalex.org/I909854389 |
| primary_location.source.host_organization_name | London School of Economics and Political Science |
| primary_location.source.host_organization_lineage | https://openalex.org/I909854389 |
| primary_location.license | other-oa |
| primary_location.pdf_url | http://eprints.lse.ac.uk/103017/1/s13063_019_3766_9.pdf |
| primary_location.version | acceptedVersion |
| primary_location.raw_type | PeerReviewed |
| primary_location.license_id | https://openalex.org/licenses/other-oa |
| primary_location.is_accepted | True |
| primary_location.is_published | False |
| primary_location.raw_source_name | |
| primary_location.landing_page_url | https://orcid.org/0000-0002-5367-9841>, |
| publication_date | 2019-12-19 |
| publication_year | 2019 |
| referenced_works_count | 0 |
| abstract_inverted_index.2 | 63, 65 |
| abstract_inverted_index.6 | 269 |
| abstract_inverted_index.a | 43, 51, 61, 176 |
| abstract_inverted_index.20 | 262 |
| abstract_inverted_index.3, | 133 |
| abstract_inverted_index.52 | 166 |
| abstract_inverted_index.6% | 80 |
| abstract_inverted_index.BE | 179, 183 |
| abstract_inverted_index.We | 34 |
| abstract_inverted_index.as | 19, 29, 172, 184, 235 |
| abstract_inverted_index.be | 109 |
| abstract_inverted_index.in | 47, 195, 223, 258 |
| abstract_inverted_index.is | 60, 159 |
| abstract_inverted_index.mg | 126, 137 |
| abstract_inverted_index.of | 6, 12, 31, 78, 92, 146, 163, 178, 203, 226 |
| abstract_inverted_index.on | 180, 261, 268 |
| abstract_inverted_index.or | 40, 42, 152, 191, 240 |
| abstract_inverted_index.to | 14, 25, 55, 70, 111, 135, 236 |
| abstract_inverted_index.× | 64 |
| abstract_inverted_index.(1) | 112 |
| abstract_inverted_index.(2) | 120 |
| abstract_inverted_index.(3) | 141 |
| abstract_inverted_index.(4) | 153 |
| abstract_inverted_index.6%) | 96 |
| abstract_inverted_index.750 | 136 |
| abstract_inverted_index.BE. | 250 |
| abstract_inverted_index.HTS | 79, 118, 149 |
| abstract_inverted_index.Key | 168 |
| abstract_inverted_index.The | 156, 229 |
| abstract_inverted_index.aid | 26 |
| abstract_inverted_index.and | 23, 75, 81, 90, 97, 115, 123, 144, 150, 189, 201, 244, 285 |
| abstract_inverted_index.are | 45, 171, 242 |
| abstract_inverted_index.day | 130 |
| abstract_inverted_index.for | 3, 83, 99, 247, 274 |
| abstract_inverted_index.per | 129, 139 |
| abstract_inverted_index.the | 4, 10, 72, 160, 185, 196, 213, 259 |
| abstract_inverted_index.two | 190 |
| abstract_inverted_index.(750 | 125 |
| abstract_inverted_index.(BE) | 8 |
| abstract_inverted_index.(HTS | 39, 95 |
| abstract_inverted_index.Care | 287 |
| abstract_inverted_index.HTS, | 238 |
| abstract_inverted_index.This | 59, 207 |
| abstract_inverted_index.V3.0 | 295 |
| abstract_inverted_index.both | 241 |
| abstract_inverted_index.care | 104, 114, 122, 143 |
| abstract_inverted_index.cost | 76, 245 |
| abstract_inverted_index.lack | 11 |
| abstract_inverted_index.last | 197 |
| abstract_inverted_index.mean | 161 |
| abstract_inverted_index.more | 192 |
| abstract_inverted_index.over | 50, 165 |
| abstract_inverted_index.part | 30 |
| abstract_inverted_index.such | 18 |
| abstract_inverted_index.that | 36 |
| abstract_inverted_index.will | 108, 232 |
| abstract_inverted_index.with | 175, 217, 249 |
| abstract_inverted_index.year | 198 |
| abstract_inverted_index.(6%), | 119 |
| abstract_inverted_index.(HTS) | 22 |
| abstract_inverted_index.CLEAR | 230 |
| abstract_inverted_index.Trial | 251 |
| abstract_inverted_index.areas | 225 |
| abstract_inverted_index.care. | 33, 57, 155 |
| abstract_inverted_index.costs | 215 |
| abstract_inverted_index.daily | 204 |
| abstract_inverted_index.day), | 140 |
| abstract_inverted_index.other | 224 |
| abstract_inverted_index.three | 127 |
| abstract_inverted_index.times | 128 |
| abstract_inverted_index.trial | 69, 227, 231 |
| abstract_inverted_index.twice | 138 |
| abstract_inverted_index.until | 131 |
| abstract_inverted_index.usual | 56, 87, 103 |
| abstract_inverted_index.visit | 132 |
| abstract_inverted_index.(first | 256 |
| abstract_inverted_index.2017). | 264 |
| abstract_inverted_index.Adults | 174 |
| abstract_inverted_index.Ethics | 289 |
| abstract_inverted_index.Health | 275, 277, 284 |
| abstract_inverted_index.Social | 286 |
| abstract_inverted_index.Trust. | 288 |
| abstract_inverted_index.agents | 38 |
| abstract_inverted_index.airway | 84, 100 |
| abstract_inverted_index.avoids | 212 |
| abstract_inverted_index.design | 211 |
| abstract_inverted_index.number | 162 |
| abstract_inverted_index.rigour | 222 |
| abstract_inverted_index.saline | 21, 94 |
| abstract_inverted_index.sputum | 27 |
| abstract_inverted_index.trial. | 106 |
| abstract_inverted_index.trials | 219 |
| abstract_inverted_index.versus | 86, 102 |
| abstract_inverted_index.weeks. | 167 |
| abstract_inverted_index.whilst | 220 |
| abstract_inverted_index.(CLEAR) | 105 |
| abstract_inverted_index.52-week | 52 |
| abstract_inverted_index.Belfast | 283 |
| abstract_inverted_index.Current | 1 |
| abstract_inverted_index.EudraCT | 253 |
| abstract_inverted_index.Funder: | 271 |
| abstract_inverted_index.Number: | 291 |
| abstract_inverted_index.October | 263 |
| abstract_inverted_index.agents, | 17 |
| abstract_inverted_index.entered | 257 |
| abstract_inverted_index.number: | 254 |
| abstract_inverted_index.outcome | 158 |
| abstract_inverted_index.period, | 53 |
| abstract_inverted_index.primary | 157, 186 |
| abstract_inverted_index.provide | 233 |
| abstract_inverted_index.removal | 28 |
| abstract_inverted_index.sputum. | 205 |
| abstract_inverted_index.trial's | 208 |
| abstract_inverted_index.whether | 237 |
| abstract_inverted_index.52-week, | 62 |
| abstract_inverted_index.Clinical | 89 |
| abstract_inverted_index.Methods: | 58 |
| abstract_inverted_index.National | 272 |
| abstract_inverted_index.Patients | 107 |
| abstract_inverted_index.Protocol | 293 |
| abstract_inverted_index.Sponsor: | 282 |
| abstract_inverted_index.care-the | 88 |
| abstract_inverted_index.clinical | 73 |
| abstract_inverted_index.compared | 54 |
| abstract_inverted_index.computed | 181 |
| abstract_inverted_index.criteria | 170 |
| abstract_inverted_index.database | 260 |
| abstract_inverted_index.evidence | 13, 234 |
| abstract_inverted_index.follows: | 173 |
| abstract_inverted_index.patients | 248 |
| abstract_inverted_index.reducing | 48, 134 |
| abstract_inverted_index.research | 210 |
| abstract_inverted_index.standard | 32, 113, 121, 142, 154 |
| abstract_inverted_index.version: | 294 |
| abstract_inverted_index.Institute | 273 |
| abstract_inverted_index.Programme | 280 |
| abstract_inverted_index.Reference | 290 |
| abstract_inverted_index.Research, | 276 |
| abstract_inverted_index.clearance | 85, 101 |
| abstract_inverted_index.delivery. | 228 |
| abstract_inverted_index.determine | 71 |
| abstract_inverted_index.diagnosis | 177 |
| abstract_inverted_index.effective | 46, 243, 246 |
| abstract_inverted_index.highlight | 9 |
| abstract_inverted_index.inclusion | 169 |
| abstract_inverted_index.nebulised | 117, 148 |
| abstract_inverted_index.pragmatic | 209 |
| abstract_inverted_index.pulmonary | 193 |
| abstract_inverted_index.recommend | 15 |
| abstract_inverted_index.requiring | 199 |
| abstract_inverted_index.Assessment | 279 |
| abstract_inverted_index.July/2018. | 270 |
| abstract_inverted_index.Registered | 267 |
| abstract_inverted_index.Technology | 278 |
| abstract_inverted_index.associated | 216 |
| abstract_inverted_index.diagnosis, | 188 |
| abstract_inverted_index.factorial, | 66 |
| abstract_inverted_index.guidelines | 2 |
| abstract_inverted_index.hypertonic | 20, 93 |
| abstract_inverted_index.management | 5 |
| abstract_inverted_index.mucoactive | 16, 37 |
| abstract_inverted_index.open-label | 68 |
| abstract_inverted_index.optimising | 221 |
| abstract_inverted_index.production | 202 |
| abstract_inverted_index.randomised | 110 |
| abstract_inverted_index.17/NE/0339. | 292 |
| abstract_inverted_index.Background: | 0 |
| abstract_inverted_index.Discussion: | 206 |
| abstract_inverted_index.ISRCTN.com, | 265 |
| abstract_inverted_index.antibiotics | 200 |
| abstract_inverted_index.combination | 145 |
| abstract_inverted_index.hypothesise | 35 |
| abstract_inverted_index.randomized, | 67 |
| abstract_inverted_index.respiratory | 187 |
| abstract_inverted_index.significant | 214 |
| abstract_inverted_index.tomography, | 182 |
| abstract_inverted_index.twice-daily | 116, 147 |
| abstract_inverted_index.(15/100/01). | 281 |
| abstract_inverted_index.combination) | 44 |
| abstract_inverted_index.double-blind | 218 |
| abstract_inverted_index.carbocisteine | 82, 98, 124, 239 |
| abstract_inverted_index.effectiveness | 74, 77 |
| abstract_inverted_index.exacerbations | 49, 164, 194 |
| abstract_inverted_index.registration: | 252 |
| abstract_inverted_index.2017-000664-14 | 255 |
| abstract_inverted_index.bronchiectasis | 7 |
| abstract_inverted_index.carbocisteine, | 24, 41, 151 |
| abstract_inverted_index.Final_14052018. | 296 |
| abstract_inverted_index.ISRCTN89040295. | 266 |
| abstract_inverted_index.cost-effectiveness | 91 |
| cited_by_percentile_year | |
| countries_distinct_count | 0 |
| institutions_distinct_count | 21 |
| citation_normalized_percentile.value | 0.33656431 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |