A low-temperature, one-step synthesis for monazite can transform monazite into a readily usable advanced nuclear waste form Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1126/sciadv.adt3518
· OA: W4408696256
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1126/sciadv.adt3518
· OA: W4408696256
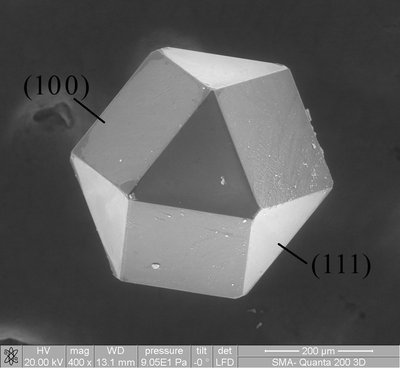
It has been demonstrated that monazite-type materials are excellent candidates for nuclear waste forms, and hence, their facile synthesis is of great importance for the needed sequestration of existing nuclear waste. The synthesis of monazite, LaPO 4 , requires inconveniently high temperatures near 1000°C and generally involves the conversion of the presynthesized rhabdophane, LaPO 4 •nH 2 O, to the LaPO 4 monazite phase. During this structure transformation, the rhabdophane converts irreversibly to the thermodynamically stable monoclinic monazite structure. A low-temperature (185° to 260°C) mild hydrothermal acid-promoted synthesis of monazite is described that can both transform presynthesized rhabdophane or assemble reagents to the monoclinic monazite structure. The pH dependence of this reaction is detailed, and its applicability to the Ln PO 4 ( Ln = La, Ce, Pr, Nd, Sm-Gd), Ca 0.5 Th 0.5 PO 4 , and Sr 0.5 Th 0.5 PO 4 systems is discussed. The crystal growth of Ca 0.5 Th 0.5 PO 4 and Sr 0.5 Th 0.5 PO 4 is described, and their crystal structures were reported. In situ x-ray diffraction studies, performed as a function of temperature, provide insight into the structure transformation process.