Abstract 2008 The Latent State of Protease Domain in Coagulation Factor IXa Discovered by X-Ray Crystallography Provides Exosite-Mediated Allosteric Control of Blood Clotting Article Swipe
 YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1016/j.jbc.2024.106217
YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1016/j.jbc.2024.106217
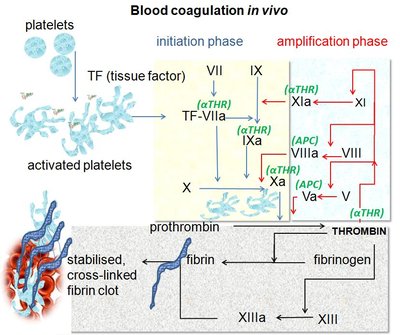
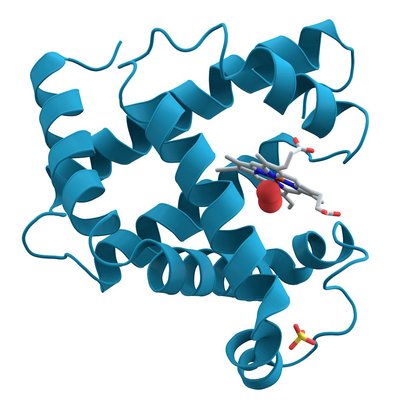
Background: Serine proteases of blood coagulation are specifically modulated by cofactors to enable timely hemostatic response without inducing thrombosis. Coagulation factor IXa, a protease component of the enzymic complex called 'intrinsic Xase', is an enigmatic enzyme due to extremely low catalytic activity in the free state. Although its protease domain has the same architecture of an activated trypsin-like protease as homologous coagulation enzymes thrombin and factor Xa, the mechanism of factor IXa function remains elusive. Objective: to study the conformational ensemble of factor IXa and reveal structural transitions enabling the gain and loss of the catalytic function. Methods: X-ray crystallography was used to resolve the structures of recombinant human factor IXa (S195A) with truncated N-terminal domains that was purified from stable AV12 cell clones. Binding affinity to biotinylated anti-IXa aptamer was measured by bio-layer interferometry in conditions with negligible mass-transport effects. Affinity of the IXa-aptamer interaction in solution was measured by fluorescence anisotropy. Active site function was assessed by kinetic measurements of fluorogenic substrate cleavage and by formation of covalent complex with antithrombin. Results: Structure of the apo-enzyme is resolved at 1.9 Å. Co-crystal structure with anti-IXa RNA aptamer having anticoagulant effect is resolved at 2.6 Å. In the apo-state, the active site of factor IXa is found in the latent conformation, with W215 side chain interfering with the entrance into S1 substrate-binding pocket. In comparison, structures of apo- thrombin and factor Xa both exhibit the open conformation of the S1 pocket, which explains a uniquely low catalytic turnover by IXa in comparison to homologous proteases. The co-crystal structure of IXa-aptamer complex shows that the aptamer does not occlude the active site and binds the protease domain in the remote exosite region, which was previously implicated in binding cofactor VIIIa and anticoagulant drug heparin. The structure shows that residues K132, L162, R165, R233 of factor IXa are important for the interaction with aptamer. Together with previous mutagenesis data, this indicates the determinants of sub-nanomolar affinity and exclusive specificity of the aptamer to IXa. Comparison of the active site conformations in apo- vs. aptamer-bound vs. substrate-bound states of factor IXa demonstrates the structural mechanism explaining the inhibitory action of the aptamer. The RNA aptamer acts allosterically by causing a 7-Å displacement of W215 that leads to the closure of S1 pocket. These structural rearrangements correlate with the aptamer-mediated inhibition of both substrate cleavage and active site-mediated interaction with antithrombin. Conclusions: This work provides the first structural evidence of allosteric control of enzymatic activity in factor IXa. The molecular surface occupied by RNA aptamer reveals an important exosite for the specific recognition and regulation of the enzymic complex assembly and catalysis. Supported by the known exosite mutations causing either bleeding or thrombotic phenotype, these findings suggest the importance of the exosite to allosterically modulate active site function of factor IXa and control the hemostatic balance. The work was supported by grant P01HL139420 from the NHLBI to S.K. and B.A.S. X-ray diffraction data were collected at the 17-ID-1 beamline at the NSLS II, a U.S. DOE Office of Science User Facility and the NE-CAT 24-ID-C beamline funded by NIH grant GM103403 with a Pilatus 6M detector funded by a NIH-ORIP HEI grant (RR029205).
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1016/j.jbc.2024.106217
- http://www.jbc.org/article/S0021925824005933/pdf
- OA Status
- gold
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4393166850
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4393166850Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1016/j.jbc.2024.106217Digital Object Identifier
- Title
-
Abstract 2008 The Latent State of Protease Domain in Coagulation Factor IXa Discovered by X-Ray Crystallography Provides Exosite-Mediated Allosteric Control of Blood ClottingWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2024Year of publication
- Publication date
-
2024-03-01Full publication date if available
- Authors
-
Vladimir N. Kolyadko, Juliana M. Layzer, Bruce A. Sullenger, Sriram KrishnaswamyList of authors in order
- Landing page
-
https://doi.org/10.1016/j.jbc.2024.106217Publisher landing page
- PDF URL
-
https://www.jbc.org/article/S0021925824005933/pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://www.jbc.org/article/S0021925824005933/pdfDirect OA link when available
- Concepts
-
Factor IXa, Allosteric regulation, Coagulation, Factor IX, Blood clotting, Factor X, Chemistry, Crystallography, Biochemistry, Biology, Medicine, Enzyme, Internal medicine, Thrombin, Platelet, ImmunologyTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4393166850 |
|---|---|
| doi | https://doi.org/10.1016/j.jbc.2024.106217 |
| ids.doi | https://doi.org/10.1016/j.jbc.2024.106217 |
| ids.openalex | https://openalex.org/W4393166850 |
| fwci | 0.0 |
| type | article |
| title | Abstract 2008 The Latent State of Protease Domain in Coagulation Factor IXa Discovered by X-Ray Crystallography Provides Exosite-Mediated Allosteric Control of Blood Clotting |
| biblio.issue | 3 |
| biblio.volume | 300 |
| biblio.last_page | 106217 |
| biblio.first_page | 106217 |
| topics[0].id | https://openalex.org/T10881 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9988999962806702 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2720 |
| topics[0].subfield.display_name | Hematology |
| topics[0].display_name | Blood Coagulation and Thrombosis Mechanisms |
| topics[1].id | https://openalex.org/T11280 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9958999752998352 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2720 |
| topics[1].subfield.display_name | Hematology |
| topics[1].display_name | Hemophilia Treatment and Research |
| topics[2].id | https://openalex.org/T11721 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9925000071525574 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2740 |
| topics[2].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[2].display_name | Blood properties and coagulation |
| is_xpac | False |
| apc_list.value | 2500 |
| apc_list.currency | USD |
| apc_list.value_usd | 2500 |
| apc_paid.value | 2500 |
| apc_paid.currency | USD |
| apc_paid.value_usd | 2500 |
| concepts[0].id | https://openalex.org/C2910886357 |
| concepts[0].level | 5 |
| concepts[0].score | 0.7872940301895142 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q425360 |
| concepts[0].display_name | Factor IXa |
| concepts[1].id | https://openalex.org/C166342909 |
| concepts[1].level | 3 |
| concepts[1].score | 0.7834084033966064 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q845326 |
| concepts[1].display_name | Allosteric regulation |
| concepts[2].id | https://openalex.org/C2778382381 |
| concepts[2].level | 2 |
| concepts[2].score | 0.6032203435897827 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q179217 |
| concepts[2].display_name | Coagulation |
| concepts[3].id | https://openalex.org/C2781221834 |
| concepts[3].level | 2 |
| concepts[3].score | 0.5186909437179565 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q425360 |
| concepts[3].display_name | Factor IX |
| concepts[4].id | https://openalex.org/C2992598832 |
| concepts[4].level | 2 |
| concepts[4].score | 0.4924331605434418 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q179217 |
| concepts[4].display_name | Blood clotting |
| concepts[5].id | https://openalex.org/C2777444498 |
| concepts[5].level | 4 |
| concepts[5].score | 0.47518789768218994 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q423701 |
| concepts[5].display_name | Factor X |
| concepts[6].id | https://openalex.org/C185592680 |
| concepts[6].level | 0 |
| concepts[6].score | 0.4087470769882202 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q2329 |
| concepts[6].display_name | Chemistry |
| concepts[7].id | https://openalex.org/C8010536 |
| concepts[7].level | 1 |
| concepts[7].score | 0.32538992166519165 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q160398 |
| concepts[7].display_name | Crystallography |
| concepts[8].id | https://openalex.org/C55493867 |
| concepts[8].level | 1 |
| concepts[8].score | 0.2392856478691101 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q7094 |
| concepts[8].display_name | Biochemistry |
| concepts[9].id | https://openalex.org/C86803240 |
| concepts[9].level | 0 |
| concepts[9].score | 0.23642858862876892 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q420 |
| concepts[9].display_name | Biology |
| concepts[10].id | https://openalex.org/C71924100 |
| concepts[10].level | 0 |
| concepts[10].score | 0.1960635483264923 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[10].display_name | Medicine |
| concepts[11].id | https://openalex.org/C181199279 |
| concepts[11].level | 2 |
| concepts[11].score | 0.15895229578018188 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q8047 |
| concepts[11].display_name | Enzyme |
| concepts[12].id | https://openalex.org/C126322002 |
| concepts[12].level | 1 |
| concepts[12].score | 0.11373105645179749 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[12].display_name | Internal medicine |
| concepts[13].id | https://openalex.org/C2777292125 |
| concepts[13].level | 3 |
| concepts[13].score | 0.11117199063301086 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q409166 |
| concepts[13].display_name | Thrombin |
| concepts[14].id | https://openalex.org/C89560881 |
| concepts[14].level | 2 |
| concepts[14].score | 0.10636153817176819 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q101026 |
| concepts[14].display_name | Platelet |
| concepts[15].id | https://openalex.org/C203014093 |
| concepts[15].level | 1 |
| concepts[15].score | 0.07983353734016418 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q101929 |
| concepts[15].display_name | Immunology |
| keywords[0].id | https://openalex.org/keywords/factor-ixa |
| keywords[0].score | 0.7872940301895142 |
| keywords[0].display_name | Factor IXa |
| keywords[1].id | https://openalex.org/keywords/allosteric-regulation |
| keywords[1].score | 0.7834084033966064 |
| keywords[1].display_name | Allosteric regulation |
| keywords[2].id | https://openalex.org/keywords/coagulation |
| keywords[2].score | 0.6032203435897827 |
| keywords[2].display_name | Coagulation |
| keywords[3].id | https://openalex.org/keywords/factor-ix |
| keywords[3].score | 0.5186909437179565 |
| keywords[3].display_name | Factor IX |
| keywords[4].id | https://openalex.org/keywords/blood-clotting |
| keywords[4].score | 0.4924331605434418 |
| keywords[4].display_name | Blood clotting |
| keywords[5].id | https://openalex.org/keywords/factor-x |
| keywords[5].score | 0.47518789768218994 |
| keywords[5].display_name | Factor X |
| keywords[6].id | https://openalex.org/keywords/chemistry |
| keywords[6].score | 0.4087470769882202 |
| keywords[6].display_name | Chemistry |
| keywords[7].id | https://openalex.org/keywords/crystallography |
| keywords[7].score | 0.32538992166519165 |
| keywords[7].display_name | Crystallography |
| keywords[8].id | https://openalex.org/keywords/biochemistry |
| keywords[8].score | 0.2392856478691101 |
| keywords[8].display_name | Biochemistry |
| keywords[9].id | https://openalex.org/keywords/biology |
| keywords[9].score | 0.23642858862876892 |
| keywords[9].display_name | Biology |
| keywords[10].id | https://openalex.org/keywords/medicine |
| keywords[10].score | 0.1960635483264923 |
| keywords[10].display_name | Medicine |
| keywords[11].id | https://openalex.org/keywords/enzyme |
| keywords[11].score | 0.15895229578018188 |
| keywords[11].display_name | Enzyme |
| keywords[12].id | https://openalex.org/keywords/internal-medicine |
| keywords[12].score | 0.11373105645179749 |
| keywords[12].display_name | Internal medicine |
| keywords[13].id | https://openalex.org/keywords/thrombin |
| keywords[13].score | 0.11117199063301086 |
| keywords[13].display_name | Thrombin |
| keywords[14].id | https://openalex.org/keywords/platelet |
| keywords[14].score | 0.10636153817176819 |
| keywords[14].display_name | Platelet |
| keywords[15].id | https://openalex.org/keywords/immunology |
| keywords[15].score | 0.07983353734016418 |
| keywords[15].display_name | Immunology |
| language | en |
| locations[0].id | doi:10.1016/j.jbc.2024.106217 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S140251998 |
| locations[0].source.issn | 0021-9258, 1067-8816, 1083-351X |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 0021-9258 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Journal of Biological Chemistry |
| locations[0].source.host_organization | https://openalex.org/P4310320990 |
| locations[0].source.host_organization_name | Elsevier BV |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320990 |
| locations[0].source.host_organization_lineage_names | Elsevier BV |
| locations[0].license | cc-by |
| locations[0].pdf_url | http://www.jbc.org/article/S0021925824005933/pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Journal of Biological Chemistry |
| locations[0].landing_page_url | https://doi.org/10.1016/j.jbc.2024.106217 |
| indexed_in | crossref, doaj |
| authorships[0].author.id | https://openalex.org/A5067681836 |
| authorships[0].author.orcid | https://orcid.org/0000-0003-4008-2461 |
| authorships[0].author.display_name | Vladimir N. Kolyadko |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I1335321130 |
| authorships[0].affiliations[0].raw_affiliation_string | The Children's Hospital of Philadelphia |
| authorships[0].institutions[0].id | https://openalex.org/I1335321130 |
| authorships[0].institutions[0].ror | https://ror.org/01z7r7q48 |
| authorships[0].institutions[0].type | healthcare |
| authorships[0].institutions[0].lineage | https://openalex.org/I1335321130 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | Children's Hospital of Philadelphia |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Vladimir Kolyadko |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | The Children's Hospital of Philadelphia |
| authorships[1].author.id | https://openalex.org/A5082046353 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Juliana M. Layzer |
| authorships[1].countries | US |
| authorships[1].affiliations[0].raw_affiliation_string | State University |
| authorships[1].affiliations[1].institution_ids | https://openalex.org/I2800191209 |
| authorships[1].affiliations[1].raw_affiliation_string | Hospital of Philadelphia |
| authorships[1].affiliations[2].institution_ids | https://openalex.org/I160856358 |
| authorships[1].affiliations[2].raw_affiliation_string | University of San Diego |
| authorships[1].institutions[0].id | https://openalex.org/I2800191209 |
| authorships[1].institutions[0].ror | https://ror.org/03mvdc478 |
| authorships[1].institutions[0].type | healthcare |
| authorships[1].institutions[0].lineage | https://openalex.org/I2800191209, https://openalex.org/I4210143392 |
| authorships[1].institutions[0].country_code | US |
| authorships[1].institutions[0].display_name | Pennsylvania Hospital |
| authorships[1].institutions[1].id | https://openalex.org/I160856358 |
| authorships[1].institutions[1].ror | https://ror.org/03jbbze48 |
| authorships[1].institutions[1].type | education |
| authorships[1].institutions[1].lineage | https://openalex.org/I160856358 |
| authorships[1].institutions[1].country_code | US |
| authorships[1].institutions[1].display_name | University of San Diego |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Juliana Layzer |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Hospital of Philadelphia, State University, University of San Diego |
| authorships[2].author.id | https://openalex.org/A5040370918 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-5113-5541 |
| authorships[2].author.display_name | Bruce A. Sullenger |
| authorships[2].countries | US |
| authorships[2].affiliations[0].raw_affiliation_string | State University |
| authorships[2].affiliations[1].institution_ids | https://openalex.org/I2800191209 |
| authorships[2].affiliations[1].raw_affiliation_string | Hospital of Philadelphia |
| authorships[2].affiliations[2].institution_ids | https://openalex.org/I160856358 |
| authorships[2].affiliations[2].raw_affiliation_string | University of San Diego |
| authorships[2].institutions[0].id | https://openalex.org/I2800191209 |
| authorships[2].institutions[0].ror | https://ror.org/03mvdc478 |
| authorships[2].institutions[0].type | healthcare |
| authorships[2].institutions[0].lineage | https://openalex.org/I2800191209, https://openalex.org/I4210143392 |
| authorships[2].institutions[0].country_code | US |
| authorships[2].institutions[0].display_name | Pennsylvania Hospital |
| authorships[2].institutions[1].id | https://openalex.org/I160856358 |
| authorships[2].institutions[1].ror | https://ror.org/03jbbze48 |
| authorships[2].institutions[1].type | education |
| authorships[2].institutions[1].lineage | https://openalex.org/I160856358 |
| authorships[2].institutions[1].country_code | US |
| authorships[2].institutions[1].display_name | University of San Diego |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Bruce Sullenger |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Hospital of Philadelphia, State University, University of San Diego |
| authorships[3].author.id | https://openalex.org/A5036209779 |
| authorships[3].author.orcid | https://orcid.org/0000-0001-6381-4325 |
| authorships[3].author.display_name | Sriram Krishnaswamy |
| authorships[3].countries | US |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I2800191209 |
| authorships[3].affiliations[0].raw_affiliation_string | Hospital of Philadelphia |
| authorships[3].affiliations[1].raw_affiliation_string | State University |
| authorships[3].affiliations[2].institution_ids | https://openalex.org/I160856358 |
| authorships[3].affiliations[2].raw_affiliation_string | University of San Diego |
| authorships[3].institutions[0].id | https://openalex.org/I2800191209 |
| authorships[3].institutions[0].ror | https://ror.org/03mvdc478 |
| authorships[3].institutions[0].type | healthcare |
| authorships[3].institutions[0].lineage | https://openalex.org/I2800191209, https://openalex.org/I4210143392 |
| authorships[3].institutions[0].country_code | US |
| authorships[3].institutions[0].display_name | Pennsylvania Hospital |
| authorships[3].institutions[1].id | https://openalex.org/I160856358 |
| authorships[3].institutions[1].ror | https://ror.org/03jbbze48 |
| authorships[3].institutions[1].type | education |
| authorships[3].institutions[1].lineage | https://openalex.org/I160856358 |
| authorships[3].institutions[1].country_code | US |
| authorships[3].institutions[1].display_name | University of San Diego |
| authorships[3].author_position | last |
| authorships[3].raw_author_name | Sriram Krishnaswamy |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Hospital of Philadelphia, State University, University of San Diego |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | http://www.jbc.org/article/S0021925824005933/pdf |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Abstract 2008 The Latent State of Protease Domain in Coagulation Factor IXa Discovered by X-Ray Crystallography Provides Exosite-Mediated Allosteric Control of Blood Clotting |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10881 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9988999962806702 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2720 |
| primary_topic.subfield.display_name | Hematology |
| primary_topic.display_name | Blood Coagulation and Thrombosis Mechanisms |
| related_works | https://openalex.org/W2025200675, https://openalex.org/W4250920934, https://openalex.org/W193982181, https://openalex.org/W1717444018, https://openalex.org/W2073530895, https://openalex.org/W2400031241, https://openalex.org/W2036019800, https://openalex.org/W2884003446, https://openalex.org/W2041868467, https://openalex.org/W1851268604 |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | doi:10.1016/j.jbc.2024.106217 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S140251998 |
| best_oa_location.source.issn | 0021-9258, 1067-8816, 1083-351X |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 0021-9258 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Journal of Biological Chemistry |
| best_oa_location.source.host_organization | https://openalex.org/P4310320990 |
| best_oa_location.source.host_organization_name | Elsevier BV |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| best_oa_location.source.host_organization_lineage_names | Elsevier BV |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | http://www.jbc.org/article/S0021925824005933/pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Journal of Biological Chemistry |
| best_oa_location.landing_page_url | https://doi.org/10.1016/j.jbc.2024.106217 |
| primary_location.id | doi:10.1016/j.jbc.2024.106217 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S140251998 |
| primary_location.source.issn | 0021-9258, 1067-8816, 1083-351X |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 0021-9258 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Journal of Biological Chemistry |
| primary_location.source.host_organization | https://openalex.org/P4310320990 |
| primary_location.source.host_organization_name | Elsevier BV |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| primary_location.source.host_organization_lineage_names | Elsevier BV |
| primary_location.license | cc-by |
| primary_location.pdf_url | http://www.jbc.org/article/S0021925824005933/pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Journal of Biological Chemistry |
| primary_location.landing_page_url | https://doi.org/10.1016/j.jbc.2024.106217 |
| publication_date | 2024-03-01 |
| publication_year | 2024 |
| referenced_works_count | 0 |
| abstract_inverted_index.a | 22, 244, 367, 500, 519, 525 |
| abstract_inverted_index.6M | 521 |
| abstract_inverted_index.In | 197, 224 |
| abstract_inverted_index.S1 | 221, 240, 378 |
| abstract_inverted_index.Xa | 232 |
| abstract_inverted_index.an | 33, 55, 423 |
| abstract_inverted_index.as | 59 |
| abstract_inverted_index.at | 180, 194, 492, 496 |
| abstract_inverted_index.by | 9, 132, 150, 158, 166, 249, 365, 419, 440, 477, 514, 524 |
| abstract_inverted_index.in | 42, 135, 146, 208, 251, 277, 286, 339, 412 |
| abstract_inverted_index.is | 32, 178, 192, 206 |
| abstract_inverted_index.of | 3, 25, 54, 69, 81, 93, 106, 142, 161, 168, 175, 203, 227, 238, 259, 303, 322, 328, 334, 346, 357, 370, 377, 388, 406, 409, 432, 456, 465, 504 |
| abstract_inverted_index.or | 448 |
| abstract_inverted_index.to | 11, 37, 76, 102, 126, 253, 331, 374, 459, 483 |
| abstract_inverted_index.1.9 | 181 |
| abstract_inverted_index.2.6 | 195 |
| abstract_inverted_index.DOE | 502 |
| abstract_inverted_index.HEI | 527 |
| abstract_inverted_index.II, | 499 |
| abstract_inverted_index.IXa | 71, 83, 110, 205, 250, 305, 348, 467 |
| abstract_inverted_index.NIH | 515 |
| abstract_inverted_index.RNA | 187, 361, 420 |
| abstract_inverted_index.The | 256, 294, 360, 415, 473 |
| abstract_inverted_index.Xa, | 66 |
| abstract_inverted_index.and | 64, 84, 91, 165, 230, 272, 290, 325, 392, 430, 437, 468, 485, 508 |
| abstract_inverted_index.are | 6, 306 |
| abstract_inverted_index.due | 36 |
| abstract_inverted_index.for | 308, 426 |
| abstract_inverted_index.has | 50 |
| abstract_inverted_index.its | 47 |
| abstract_inverted_index.low | 39, 246 |
| abstract_inverted_index.not | 267 |
| abstract_inverted_index.the | 26, 43, 51, 67, 78, 89, 94, 104, 143, 176, 198, 200, 209, 218, 235, 239, 264, 269, 274, 278, 309, 320, 329, 335, 350, 354, 358, 375, 385, 402, 427, 433, 441, 454, 457, 470, 481, 493, 497, 509 |
| abstract_inverted_index.vs. | 341, 343 |
| abstract_inverted_index.was | 100, 117, 130, 148, 156, 283, 475 |
| abstract_inverted_index.Å. | 182, 196 |
| abstract_inverted_index.7-Å | 368 |
| abstract_inverted_index.AV12 | 121 |
| abstract_inverted_index.IXa, | 21 |
| abstract_inverted_index.IXa. | 332, 414 |
| abstract_inverted_index.NSLS | 498 |
| abstract_inverted_index.R233 | 302 |
| abstract_inverted_index.S.K. | 484 |
| abstract_inverted_index.This | 399 |
| abstract_inverted_index.U.S. | 501 |
| abstract_inverted_index.User | 506 |
| abstract_inverted_index.W215 | 213, 371 |
| abstract_inverted_index.acts | 363 |
| abstract_inverted_index.apo- | 228, 340 |
| abstract_inverted_index.both | 233, 389 |
| abstract_inverted_index.cell | 122 |
| abstract_inverted_index.data | 489 |
| abstract_inverted_index.does | 266 |
| abstract_inverted_index.drug | 292 |
| abstract_inverted_index.free | 44 |
| abstract_inverted_index.from | 119, 480 |
| abstract_inverted_index.gain | 90 |
| abstract_inverted_index.into | 220 |
| abstract_inverted_index.loss | 92 |
| abstract_inverted_index.open | 236 |
| abstract_inverted_index.same | 52 |
| abstract_inverted_index.side | 214 |
| abstract_inverted_index.site | 154, 202, 271, 337, 463 |
| abstract_inverted_index.that | 116, 263, 297, 372 |
| abstract_inverted_index.this | 318 |
| abstract_inverted_index.used | 101 |
| abstract_inverted_index.were | 490 |
| abstract_inverted_index.with | 112, 137, 171, 185, 212, 217, 311, 314, 384, 396, 518 |
| abstract_inverted_index.work | 400, 474 |
| abstract_inverted_index.K132, | 299 |
| abstract_inverted_index.L162, | 300 |
| abstract_inverted_index.NHLBI | 482 |
| abstract_inverted_index.R165, | 301 |
| abstract_inverted_index.These | 380 |
| abstract_inverted_index.VIIIa | 289 |
| abstract_inverted_index.X-ray | 98, 487 |
| abstract_inverted_index.binds | 273 |
| abstract_inverted_index.blood | 4 |
| abstract_inverted_index.chain | 215 |
| abstract_inverted_index.data, | 317 |
| abstract_inverted_index.first | 403 |
| abstract_inverted_index.found | 207 |
| abstract_inverted_index.grant | 478, 516, 528 |
| abstract_inverted_index.human | 108 |
| abstract_inverted_index.known | 442 |
| abstract_inverted_index.leads | 373 |
| abstract_inverted_index.shows | 262, 296 |
| abstract_inverted_index.study | 77 |
| abstract_inverted_index.these | 451 |
| abstract_inverted_index.which | 242, 282 |
| abstract_inverted_index.Active | 153 |
| abstract_inverted_index.B.A.S. | 486 |
| abstract_inverted_index.NE-CAT | 510 |
| abstract_inverted_index.Office | 503 |
| abstract_inverted_index.Serine | 1 |
| abstract_inverted_index.Xase', | 31 |
| abstract_inverted_index.action | 356 |
| abstract_inverted_index.active | 201, 270, 336, 393, 462 |
| abstract_inverted_index.called | 29 |
| abstract_inverted_index.domain | 49, 276 |
| abstract_inverted_index.effect | 191 |
| abstract_inverted_index.either | 446 |
| abstract_inverted_index.enable | 12 |
| abstract_inverted_index.enzyme | 35 |
| abstract_inverted_index.factor | 20, 65, 70, 82, 109, 204, 231, 304, 347, 413, 466 |
| abstract_inverted_index.funded | 513, 523 |
| abstract_inverted_index.having | 189 |
| abstract_inverted_index.latent | 210 |
| abstract_inverted_index.remote | 279 |
| abstract_inverted_index.reveal | 85 |
| abstract_inverted_index.stable | 120 |
| abstract_inverted_index.state. | 45 |
| abstract_inverted_index.states | 345 |
| abstract_inverted_index.timely | 13 |
| abstract_inverted_index.(S195A) | 111 |
| abstract_inverted_index.17-ID-1 | 494 |
| abstract_inverted_index.24-ID-C | 511 |
| abstract_inverted_index.Binding | 124 |
| abstract_inverted_index.Pilatus | 520 |
| abstract_inverted_index.Science | 505 |
| abstract_inverted_index.aptamer | 129, 188, 265, 330, 362, 421 |
| abstract_inverted_index.binding | 287 |
| abstract_inverted_index.causing | 366, 445 |
| abstract_inverted_index.clones. | 123 |
| abstract_inverted_index.closure | 376 |
| abstract_inverted_index.complex | 28, 170, 261, 435 |
| abstract_inverted_index.control | 408, 469 |
| abstract_inverted_index.domains | 115 |
| abstract_inverted_index.enzymes | 62 |
| abstract_inverted_index.enzymic | 27, 434 |
| abstract_inverted_index.exhibit | 234 |
| abstract_inverted_index.exosite | 280, 425, 443, 458 |
| abstract_inverted_index.kinetic | 159 |
| abstract_inverted_index.occlude | 268 |
| abstract_inverted_index.pocket, | 241 |
| abstract_inverted_index.pocket. | 223, 379 |
| abstract_inverted_index.region, | 281 |
| abstract_inverted_index.remains | 73 |
| abstract_inverted_index.resolve | 103 |
| abstract_inverted_index.reveals | 422 |
| abstract_inverted_index.suggest | 453 |
| abstract_inverted_index.surface | 417 |
| abstract_inverted_index.without | 16 |
| abstract_inverted_index.Affinity | 141 |
| abstract_inverted_index.Although | 46 |
| abstract_inverted_index.Facility | 507 |
| abstract_inverted_index.GM103403 | 517 |
| abstract_inverted_index.Methods: | 97 |
| abstract_inverted_index.NIH-ORIP | 526 |
| abstract_inverted_index.Results: | 173 |
| abstract_inverted_index.Together | 313 |
| abstract_inverted_index.activity | 41, 411 |
| abstract_inverted_index.affinity | 125, 324 |
| abstract_inverted_index.anti-IXa | 128, 186 |
| abstract_inverted_index.aptamer. | 312, 359 |
| abstract_inverted_index.assembly | 436 |
| abstract_inverted_index.assessed | 157 |
| abstract_inverted_index.balance. | 472 |
| abstract_inverted_index.beamline | 495, 512 |
| abstract_inverted_index.bleeding | 447 |
| abstract_inverted_index.cleavage | 164, 391 |
| abstract_inverted_index.cofactor | 288 |
| abstract_inverted_index.covalent | 169 |
| abstract_inverted_index.detector | 522 |
| abstract_inverted_index.effects. | 140 |
| abstract_inverted_index.elusive. | 74 |
| abstract_inverted_index.enabling | 88 |
| abstract_inverted_index.ensemble | 80 |
| abstract_inverted_index.entrance | 219 |
| abstract_inverted_index.evidence | 405 |
| abstract_inverted_index.explains | 243 |
| abstract_inverted_index.findings | 452 |
| abstract_inverted_index.function | 72, 155, 464 |
| abstract_inverted_index.heparin. | 293 |
| abstract_inverted_index.inducing | 17 |
| abstract_inverted_index.measured | 131, 149 |
| abstract_inverted_index.modulate | 461 |
| abstract_inverted_index.occupied | 418 |
| abstract_inverted_index.previous | 315 |
| abstract_inverted_index.protease | 23, 48, 58, 275 |
| abstract_inverted_index.provides | 401 |
| abstract_inverted_index.purified | 118 |
| abstract_inverted_index.residues | 298 |
| abstract_inverted_index.resolved | 179, 193 |
| abstract_inverted_index.response | 15 |
| abstract_inverted_index.solution | 147 |
| abstract_inverted_index.specific | 428 |
| abstract_inverted_index.thrombin | 63, 229 |
| abstract_inverted_index.turnover | 248 |
| abstract_inverted_index.uniquely | 245 |
| abstract_inverted_index.Structure | 174 |
| abstract_inverted_index.Supported | 439 |
| abstract_inverted_index.activated | 56 |
| abstract_inverted_index.bio-layer | 133 |
| abstract_inverted_index.catalytic | 40, 95, 247 |
| abstract_inverted_index.cofactors | 10 |
| abstract_inverted_index.collected | 491 |
| abstract_inverted_index.component | 24 |
| abstract_inverted_index.correlate | 383 |
| abstract_inverted_index.enigmatic | 34 |
| abstract_inverted_index.enzymatic | 410 |
| abstract_inverted_index.exclusive | 326 |
| abstract_inverted_index.extremely | 38 |
| abstract_inverted_index.formation | 167 |
| abstract_inverted_index.function. | 96 |
| abstract_inverted_index.important | 307, 424 |
| abstract_inverted_index.indicates | 319 |
| abstract_inverted_index.mechanism | 68, 352 |
| abstract_inverted_index.modulated | 8 |
| abstract_inverted_index.molecular | 416 |
| abstract_inverted_index.mutations | 444 |
| abstract_inverted_index.proteases | 2 |
| abstract_inverted_index.structure | 184, 258, 295 |
| abstract_inverted_index.substrate | 163, 390 |
| abstract_inverted_index.supported | 476 |
| abstract_inverted_index.truncated | 113 |
| abstract_inverted_index.'intrinsic | 30 |
| abstract_inverted_index.Co-crystal | 183 |
| abstract_inverted_index.Comparison | 333 |
| abstract_inverted_index.N-terminal | 114 |
| abstract_inverted_index.Objective: | 75 |
| abstract_inverted_index.allosteric | 407 |
| abstract_inverted_index.apo-enzyme | 177 |
| abstract_inverted_index.apo-state, | 199 |
| abstract_inverted_index.catalysis. | 438 |
| abstract_inverted_index.co-crystal | 257 |
| abstract_inverted_index.comparison | 252 |
| abstract_inverted_index.conditions | 136 |
| abstract_inverted_index.explaining | 353 |
| abstract_inverted_index.hemostatic | 14, 471 |
| abstract_inverted_index.homologous | 60, 254 |
| abstract_inverted_index.implicated | 285 |
| abstract_inverted_index.importance | 455 |
| abstract_inverted_index.inhibition | 387 |
| abstract_inverted_index.inhibitory | 355 |
| abstract_inverted_index.negligible | 138 |
| abstract_inverted_index.phenotype, | 450 |
| abstract_inverted_index.previously | 284 |
| abstract_inverted_index.proteases. | 255 |
| abstract_inverted_index.regulation | 431 |
| abstract_inverted_index.structural | 86, 351, 381, 404 |
| abstract_inverted_index.structures | 105, 226 |
| abstract_inverted_index.thrombotic | 449 |
| abstract_inverted_index.(RR029205). | 529 |
| abstract_inverted_index.Background: | 0 |
| abstract_inverted_index.Coagulation | 19 |
| abstract_inverted_index.IXa-aptamer | 144, 260 |
| abstract_inverted_index.P01HL139420 | 479 |
| abstract_inverted_index.anisotropy. | 152 |
| abstract_inverted_index.coagulation | 5, 61 |
| abstract_inverted_index.comparison, | 225 |
| abstract_inverted_index.diffraction | 488 |
| abstract_inverted_index.fluorogenic | 162 |
| abstract_inverted_index.interaction | 145, 310, 395 |
| abstract_inverted_index.interfering | 216 |
| abstract_inverted_index.mutagenesis | 316 |
| abstract_inverted_index.recognition | 429 |
| abstract_inverted_index.recombinant | 107 |
| abstract_inverted_index.specificity | 327 |
| abstract_inverted_index.thrombosis. | 18 |
| abstract_inverted_index.transitions | 87 |
| abstract_inverted_index.Conclusions: | 398 |
| abstract_inverted_index.architecture | 53 |
| abstract_inverted_index.biotinylated | 127 |
| abstract_inverted_index.conformation | 237 |
| abstract_inverted_index.demonstrates | 349 |
| abstract_inverted_index.determinants | 321 |
| abstract_inverted_index.displacement | 369 |
| abstract_inverted_index.fluorescence | 151 |
| abstract_inverted_index.measurements | 160 |
| abstract_inverted_index.specifically | 7 |
| abstract_inverted_index.trypsin-like | 57 |
| abstract_inverted_index.anticoagulant | 190, 291 |
| abstract_inverted_index.antithrombin. | 172, 397 |
| abstract_inverted_index.aptamer-bound | 342 |
| abstract_inverted_index.conformation, | 211 |
| abstract_inverted_index.conformations | 338 |
| abstract_inverted_index.site-mediated | 394 |
| abstract_inverted_index.sub-nanomolar | 323 |
| abstract_inverted_index.allosterically | 364, 460 |
| abstract_inverted_index.conformational | 79 |
| abstract_inverted_index.interferometry | 134 |
| abstract_inverted_index.mass-transport | 139 |
| abstract_inverted_index.rearrangements | 382 |
| abstract_inverted_index.crystallography | 99 |
| abstract_inverted_index.substrate-bound | 344 |
| abstract_inverted_index.aptamer-mediated | 386 |
| abstract_inverted_index.substrate-binding | 222 |
| cited_by_percentile_year | |
| countries_distinct_count | 1 |
| institutions_distinct_count | 4 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/6 |
| sustainable_development_goals[0].score | 0.49000000953674316 |
| sustainable_development_goals[0].display_name | Clean water and sanitation |
| citation_normalized_percentile.value | 0.07115164 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |