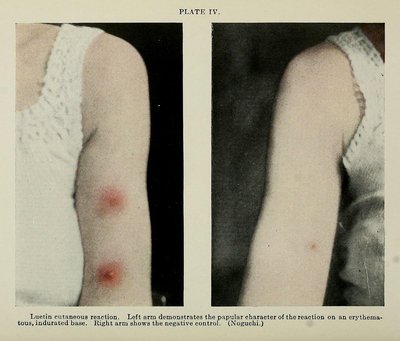
Assessment of Efficacy of Adjuvant Topical Rituximab Encapsulated in Nanoparticle Gel in Oral Pemphigus Vulgaris: A Randomized, Double-Blind, Placebo-Controlled, Pilot Study Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.4103/idoj.idoj_885_24
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.4103/idoj.idoj_885_24
Background: Oral pemphigus vulgaris (PV) presents with persistent, painful, nonhealing oral erosions, with a slower treatment response compared to cutaneous lesions. Patients and Methods: To assess the efficacy of adjuvant topical rituximab encapsulated in nanoparticle gel in oral PV. Of 31 oral PV patients recruited, 16 were randomized to rituximab incorporated in calcium alginate nanoparticles (study group) and 15 to calcium alginate nanoparticle gel only (placebo group), applied twice daily on oral erosions for 12 weeks. Both groups, in addition, received oral prednisolone (tapering doses) and azathioprine. Results: In the topical rituximab group, 75% of patients and in the placebo group, 86.7% of patients achieved remission ( P = 0.65) over 12 weeks of therapy. The median time to remission in the topical rituximab group was 4 weeks, and in the placebo group, it was 8 weeks ( P = 0.53). Median oral pemphigus scores showed an earlier reduction in the rituximab group ( P = 0.52). The median circulating CD20 count was reduced in the topical rituximab group from 100 to 74/mm 3 at week 8. In the placebo group, the value increased from 89 to 118/mm 3 (intergroup P = 0.29). A significantly greater number of cases in the topical rituximab group had a negative mucosal CD20 count ( P = 0.001). There was a comparable fall in desmoglein 3 titers in both groups ( P = 0.58). The mean cumulative prednisolone dose was 3567 mg in the topical rituximab group and 3869 mg in the placebo group ( P = 0.125). No side effects of rituximab were observed. Limitations: A small sample size, and need of pharmacokinetic studies in animal models to assess absorption of rituximab in nanogel formulation. Conclusion: With topical rituximab incorporated in nanogel, there was a trend toward earlier remission, lower total cumulative oral prednisolone dose, earlier improvement in oral pemphigus score, and greater reduction in CD20 count.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.4103/idoj.idoj_885_24
- OA Status
- diamond
- Cited By
- 1
- References
- 9
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4411493202
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4411493202Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.4103/idoj.idoj_885_24Digital Object Identifier
- Title
-
Assessment of Efficacy of Adjuvant Topical Rituximab Encapsulated in Nanoparticle Gel in Oral Pemphigus Vulgaris: A Randomized, Double-Blind, Placebo-Controlled, Pilot StudyWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-06-20Full publication date if available
- Authors
-
Pratik Mohta, Sujay Khandpur, Amit Kumar Dinda, Madhusudan Bhat, Seema Tyagi, Vinod Sharma, Mani KalaivaniList of authors in order
- Landing page
-
https://doi.org/10.4103/idoj.idoj_885_24Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
diamondOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.4103/idoj.idoj_885_24Direct OA link when available
- Concepts
-
Medicine, Pemphigus vulgaris, Rituximab, Adjuvant, Dermatology, Placebo, Double blind, Randomized controlled trial, Internal medicine, Immunology, Antibody, Pathology, Alternative medicineTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
1Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 1Per-year citation counts (last 5 years)
- References (count)
-
9Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4411493202 |
|---|---|
| doi | https://doi.org/10.4103/idoj.idoj_885_24 |
| ids.doi | https://doi.org/10.4103/idoj.idoj_885_24 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/40688121 |
| ids.openalex | https://openalex.org/W4411493202 |
| fwci | 4.90612426 |
| type | article |
| title | Assessment of Efficacy of Adjuvant Topical Rituximab Encapsulated in Nanoparticle Gel in Oral Pemphigus Vulgaris: A Randomized, Double-Blind, Placebo-Controlled, Pilot Study |
| biblio.issue | 4 |
| biblio.volume | 16 |
| biblio.last_page | 590 |
| biblio.first_page | 586 |
| topics[0].id | https://openalex.org/T11730 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 1.0 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2734 |
| topics[0].subfield.display_name | Pathology and Forensic Medicine |
| topics[0].display_name | Autoimmune Bullous Skin Diseases |
| topics[1].id | https://openalex.org/T11167 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9656000137329102 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2748 |
| topics[1].subfield.display_name | Urology |
| topics[1].display_name | Hair Growth and Disorders |
| topics[2].id | https://openalex.org/T11917 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9642999768257141 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2716 |
| topics[2].subfield.display_name | Genetics |
| topics[2].display_name | Coagulation, Bradykinin, Polyphosphates, and Angioedema |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.9363739490509033 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C2781313415 |
| concepts[1].level | 2 |
| concepts[1].score | 0.8597164750099182 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q3899001 |
| concepts[1].display_name | Pemphigus vulgaris |
| concepts[2].id | https://openalex.org/C2780653079 |
| concepts[2].level | 3 |
| concepts[2].score | 0.6869944334030151 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q412323 |
| concepts[2].display_name | Rituximab |
| concepts[3].id | https://openalex.org/C2777863537 |
| concepts[3].level | 2 |
| concepts[3].score | 0.6466012001037598 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q357896 |
| concepts[3].display_name | Adjuvant |
| concepts[4].id | https://openalex.org/C16005928 |
| concepts[4].level | 1 |
| concepts[4].score | 0.6239013075828552 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q171171 |
| concepts[4].display_name | Dermatology |
| concepts[5].id | https://openalex.org/C27081682 |
| concepts[5].level | 3 |
| concepts[5].score | 0.6205378174781799 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q269829 |
| concepts[5].display_name | Placebo |
| concepts[6].id | https://openalex.org/C2991744798 |
| concepts[6].level | 4 |
| concepts[6].score | 0.6090489625930786 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q608510 |
| concepts[6].display_name | Double blind |
| concepts[7].id | https://openalex.org/C168563851 |
| concepts[7].level | 2 |
| concepts[7].score | 0.4650880694389343 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[7].display_name | Randomized controlled trial |
| concepts[8].id | https://openalex.org/C126322002 |
| concepts[8].level | 1 |
| concepts[8].score | 0.2515588104724884 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[8].display_name | Internal medicine |
| concepts[9].id | https://openalex.org/C203014093 |
| concepts[9].level | 1 |
| concepts[9].score | 0.16699951887130737 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q101929 |
| concepts[9].display_name | Immunology |
| concepts[10].id | https://openalex.org/C159654299 |
| concepts[10].level | 2 |
| concepts[10].score | 0.1647169589996338 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q79460 |
| concepts[10].display_name | Antibody |
| concepts[11].id | https://openalex.org/C142724271 |
| concepts[11].level | 1 |
| concepts[11].score | 0.12029057741165161 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q7208 |
| concepts[11].display_name | Pathology |
| concepts[12].id | https://openalex.org/C204787440 |
| concepts[12].level | 2 |
| concepts[12].score | 0.09560203552246094 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q188504 |
| concepts[12].display_name | Alternative medicine |
| keywords[0].id | https://openalex.org/keywords/medicine |
| keywords[0].score | 0.9363739490509033 |
| keywords[0].display_name | Medicine |
| keywords[1].id | https://openalex.org/keywords/pemphigus-vulgaris |
| keywords[1].score | 0.8597164750099182 |
| keywords[1].display_name | Pemphigus vulgaris |
| keywords[2].id | https://openalex.org/keywords/rituximab |
| keywords[2].score | 0.6869944334030151 |
| keywords[2].display_name | Rituximab |
| keywords[3].id | https://openalex.org/keywords/adjuvant |
| keywords[3].score | 0.6466012001037598 |
| keywords[3].display_name | Adjuvant |
| keywords[4].id | https://openalex.org/keywords/dermatology |
| keywords[4].score | 0.6239013075828552 |
| keywords[4].display_name | Dermatology |
| keywords[5].id | https://openalex.org/keywords/placebo |
| keywords[5].score | 0.6205378174781799 |
| keywords[5].display_name | Placebo |
| keywords[6].id | https://openalex.org/keywords/double-blind |
| keywords[6].score | 0.6090489625930786 |
| keywords[6].display_name | Double blind |
| keywords[7].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[7].score | 0.4650880694389343 |
| keywords[7].display_name | Randomized controlled trial |
| keywords[8].id | https://openalex.org/keywords/internal-medicine |
| keywords[8].score | 0.2515588104724884 |
| keywords[8].display_name | Internal medicine |
| keywords[9].id | https://openalex.org/keywords/immunology |
| keywords[9].score | 0.16699951887130737 |
| keywords[9].display_name | Immunology |
| keywords[10].id | https://openalex.org/keywords/antibody |
| keywords[10].score | 0.1647169589996338 |
| keywords[10].display_name | Antibody |
| keywords[11].id | https://openalex.org/keywords/pathology |
| keywords[11].score | 0.12029057741165161 |
| keywords[11].display_name | Pathology |
| keywords[12].id | https://openalex.org/keywords/alternative-medicine |
| keywords[12].score | 0.09560203552246094 |
| keywords[12].display_name | Alternative medicine |
| language | en |
| locations[0].id | doi:10.4103/idoj.idoj_885_24 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S2754097677 |
| locations[0].source.issn | 2229-5178, 2249-5673 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 2229-5178 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Indian Dermatology Online Journal |
| locations[0].source.host_organization | https://openalex.org/P4310320448 |
| locations[0].source.host_organization_name | Medknow |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320448 |
| locations[0].license | cc-by-nc-sa |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by-nc-sa |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Indian Dermatology Online Journal |
| locations[0].landing_page_url | https://doi.org/10.4103/idoj.idoj_885_24 |
| locations[1].id | pmid:40688121 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Indian dermatology online journal |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/40688121 |
| locations[2].id | pmh:oai:doaj.org/article:22f2160d95b446c4b8ec162e635f3534 |
| locations[2].is_oa | False |
| locations[2].source.id | https://openalex.org/S4306401280 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[2].source.host_organization | |
| locations[2].source.host_organization_name | |
| locations[2].source.host_organization_lineage | |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | article |
| locations[2].license_id | |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | Indian Dermatology Online Journal, Vol 16, Iss 4, Pp 586-590 (2025) |
| locations[2].landing_page_url | https://doaj.org/article/22f2160d95b446c4b8ec162e635f3534 |
| locations[3].id | pmh:oai:pubmedcentral.nih.gov:12270421 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S2764455111 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | PubMed Central |
| locations[3].source.host_organization | https://openalex.org/I1299303238 |
| locations[3].source.host_organization_name | National Institutes of Health |
| locations[3].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[3].license | other-oa |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | Text |
| locations[3].license_id | https://openalex.org/licenses/other-oa |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | Indian Dermatol Online J |
| locations[3].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/12270421 |
| indexed_in | crossref, doaj, pubmed |
| authorships[0].author.id | https://openalex.org/A5000997288 |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | Pratik Mohta |
| authorships[0].countries | IN |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[0].affiliations[0].raw_affiliation_string | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[0].institutions[0].id | https://openalex.org/I63739035 |
| authorships[0].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[0].institutions[0].country_code | IN |
| authorships[0].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Pratik Rajendra Mohta |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[1].author.id | https://openalex.org/A5050917954 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Sujay Khandpur |
| authorships[1].countries | IN |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[1].affiliations[0].raw_affiliation_string | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[1].institutions[0].id | https://openalex.org/I63739035 |
| authorships[1].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[1].institutions[0].country_code | IN |
| authorships[1].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Sujay Khandpur |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[2].author.id | https://openalex.org/A5040811919 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-0681-1635 |
| authorships[2].author.display_name | Amit Kumar Dinda |
| authorships[2].countries | IN |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[2].affiliations[0].raw_affiliation_string | Department of Pathology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[2].institutions[0].id | https://openalex.org/I63739035 |
| authorships[2].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[2].institutions[0].type | education |
| authorships[2].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[2].institutions[0].country_code | IN |
| authorships[2].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Amit Kumar Dinda |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Department of Pathology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[3].author.id | https://openalex.org/A5057512177 |
| authorships[3].author.orcid | https://orcid.org/0000-0003-0472-8966 |
| authorships[3].author.display_name | Madhusudan Bhat |
| authorships[3].countries | IN |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[3].affiliations[0].raw_affiliation_string | Department of Pathology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[3].institutions[0].id | https://openalex.org/I63739035 |
| authorships[3].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[3].institutions[0].country_code | IN |
| authorships[3].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Madhusudan Bhat |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Department of Pathology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[4].author.id | https://openalex.org/A5105053367 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Seema Tyagi |
| authorships[4].countries | IN |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[4].affiliations[0].raw_affiliation_string | Department of Hematology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[4].institutions[0].id | https://openalex.org/I63739035 |
| authorships[4].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[4].institutions[0].type | education |
| authorships[4].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[4].institutions[0].country_code | IN |
| authorships[4].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Seema Tyagi |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Department of Hematology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[5].author.id | https://openalex.org/A5100689532 |
| authorships[5].author.orcid | https://orcid.org/0000-0002-2984-7374 |
| authorships[5].author.display_name | Vinod Sharma |
| authorships[5].countries | IN |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[5].affiliations[0].raw_affiliation_string | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[5].institutions[0].id | https://openalex.org/I63739035 |
| authorships[5].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[5].institutions[0].type | education |
| authorships[5].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[5].institutions[0].country_code | IN |
| authorships[5].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Vinod Kumar Sharma |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India |
| authorships[6].author.id | https://openalex.org/A5042411283 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-6492-1808 |
| authorships[6].author.display_name | Mani Kalaivani |
| authorships[6].countries | IN |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I63739035 |
| authorships[6].affiliations[0].raw_affiliation_string | Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India |
| authorships[6].institutions[0].id | https://openalex.org/I63739035 |
| authorships[6].institutions[0].ror | https://ror.org/02dwcqs71 |
| authorships[6].institutions[0].type | education |
| authorships[6].institutions[0].lineage | https://openalex.org/I2799351866, https://openalex.org/I4210148677, https://openalex.org/I63739035 |
| authorships[6].institutions[0].country_code | IN |
| authorships[6].institutions[0].display_name | All India Institute of Medical Sciences |
| authorships[6].author_position | last |
| authorships[6].raw_author_name | Mani Kalaivani |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.4103/idoj.idoj_885_24 |
| open_access.oa_status | diamond |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Assessment of Efficacy of Adjuvant Topical Rituximab Encapsulated in Nanoparticle Gel in Oral Pemphigus Vulgaris: A Randomized, Double-Blind, Placebo-Controlled, Pilot Study |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T11730 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 1.0 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2734 |
| primary_topic.subfield.display_name | Pathology and Forensic Medicine |
| primary_topic.display_name | Autoimmune Bullous Skin Diseases |
| related_works | https://openalex.org/W1531542607, https://openalex.org/W4387255467, https://openalex.org/W2119996668, https://openalex.org/W2414235044, https://openalex.org/W2076071554, https://openalex.org/W3089286105, https://openalex.org/W4287009336, https://openalex.org/W2091245624, https://openalex.org/W1862250341, https://openalex.org/W2902511548 |
| cited_by_count | 1 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 1 |
| locations_count | 4 |
| best_oa_location.id | doi:10.4103/idoj.idoj_885_24 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S2754097677 |
| best_oa_location.source.issn | 2229-5178, 2249-5673 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 2229-5178 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Indian Dermatology Online Journal |
| best_oa_location.source.host_organization | https://openalex.org/P4310320448 |
| best_oa_location.source.host_organization_name | Medknow |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320448 |
| best_oa_location.license | cc-by-nc-sa |
| best_oa_location.pdf_url | |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by-nc-sa |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Indian Dermatology Online Journal |
| best_oa_location.landing_page_url | https://doi.org/10.4103/idoj.idoj_885_24 |
| primary_location.id | doi:10.4103/idoj.idoj_885_24 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S2754097677 |
| primary_location.source.issn | 2229-5178, 2249-5673 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 2229-5178 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Indian Dermatology Online Journal |
| primary_location.source.host_organization | https://openalex.org/P4310320448 |
| primary_location.source.host_organization_name | Medknow |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320448 |
| primary_location.license | cc-by-nc-sa |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by-nc-sa |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Indian Dermatology Online Journal |
| primary_location.landing_page_url | https://doi.org/10.4103/idoj.idoj_885_24 |
| publication_date | 2025-06-20 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W2787771368, https://openalex.org/W2050442489, https://openalex.org/W2094543650, https://openalex.org/W2059661118, https://openalex.org/W2116207000, https://openalex.org/W2789555421, https://openalex.org/W2158280606, https://openalex.org/W2575627033, https://openalex.org/W2046814128 |
| referenced_works_count | 9 |
| abstract_inverted_index.( | 107, 138, 154, 211, 227, 251 |
| abstract_inverted_index.3 | 174, 189, 222 |
| abstract_inverted_index.4 | 127 |
| abstract_inverted_index.8 | 136 |
| abstract_inverted_index.= | 109, 140, 156, 192, 213, 229, 253 |
| abstract_inverted_index.A | 194, 263 |
| abstract_inverted_index.P | 108, 139, 155, 191, 212, 228, 252 |
| abstract_inverted_index.a | 14, 206, 217, 292 |
| abstract_inverted_index.12 | 75, 112 |
| abstract_inverted_index.15 | 59 |
| abstract_inverted_index.16 | 46 |
| abstract_inverted_index.31 | 41 |
| abstract_inverted_index.8. | 177 |
| abstract_inverted_index.89 | 186 |
| abstract_inverted_index.In | 89, 178 |
| abstract_inverted_index.No | 255 |
| abstract_inverted_index.Of | 40 |
| abstract_inverted_index.PV | 43 |
| abstract_inverted_index.To | 25 |
| abstract_inverted_index.an | 147 |
| abstract_inverted_index.at | 175 |
| abstract_inverted_index.in | 34, 37, 52, 79, 98, 121, 130, 150, 165, 200, 220, 224, 239, 247, 272, 280, 288, 305, 312 |
| abstract_inverted_index.it | 134 |
| abstract_inverted_index.mg | 238, 246 |
| abstract_inverted_index.of | 29, 95, 103, 114, 198, 258, 269, 278 |
| abstract_inverted_index.on | 71 |
| abstract_inverted_index.to | 19, 49, 60, 119, 172, 187, 275 |
| abstract_inverted_index.100 | 171 |
| abstract_inverted_index.75% | 94 |
| abstract_inverted_index.PV. | 39 |
| abstract_inverted_index.The | 116, 158, 231 |
| abstract_inverted_index.and | 23, 58, 86, 97, 129, 244, 267, 309 |
| abstract_inverted_index.for | 74 |
| abstract_inverted_index.gel | 36, 64 |
| abstract_inverted_index.had | 205 |
| abstract_inverted_index.the | 27, 90, 99, 122, 131, 151, 166, 179, 182, 201, 240, 248 |
| abstract_inverted_index.was | 126, 135, 163, 216, 236, 291 |
| abstract_inverted_index.(PV) | 5 |
| abstract_inverted_index.3567 | 237 |
| abstract_inverted_index.3869 | 245 |
| abstract_inverted_index.Both | 77 |
| abstract_inverted_index.CD20 | 161, 209, 313 |
| abstract_inverted_index.Oral | 2 |
| abstract_inverted_index.With | 284 |
| abstract_inverted_index.both | 225 |
| abstract_inverted_index.dose | 235 |
| abstract_inverted_index.fall | 219 |
| abstract_inverted_index.from | 170, 185 |
| abstract_inverted_index.mean | 232 |
| abstract_inverted_index.need | 268 |
| abstract_inverted_index.only | 65 |
| abstract_inverted_index.oral | 11, 38, 42, 72, 82, 143, 300, 306 |
| abstract_inverted_index.over | 111 |
| abstract_inverted_index.side | 256 |
| abstract_inverted_index.time | 118 |
| abstract_inverted_index.week | 176 |
| abstract_inverted_index.were | 47, 260 |
| abstract_inverted_index.with | 7, 13 |
| abstract_inverted_index.0.65) | 110 |
| abstract_inverted_index.74/mm | 173 |
| abstract_inverted_index.86.7% | 102 |
| abstract_inverted_index.There | 215 |
| abstract_inverted_index.cases | 199 |
| abstract_inverted_index.count | 162, 210 |
| abstract_inverted_index.daily | 70 |
| abstract_inverted_index.dose, | 302 |
| abstract_inverted_index.group | 125, 153, 169, 204, 243, 250 |
| abstract_inverted_index.lower | 297 |
| abstract_inverted_index.size, | 266 |
| abstract_inverted_index.small | 264 |
| abstract_inverted_index.there | 290 |
| abstract_inverted_index.total | 298 |
| abstract_inverted_index.trend | 293 |
| abstract_inverted_index.twice | 69 |
| abstract_inverted_index.value | 183 |
| abstract_inverted_index.weeks | 113, 137 |
| abstract_inverted_index.(study | 56 |
| abstract_inverted_index.0.29). | 193 |
| abstract_inverted_index.0.52). | 157 |
| abstract_inverted_index.0.53). | 141 |
| abstract_inverted_index.0.58). | 230 |
| abstract_inverted_index.118/mm | 188 |
| abstract_inverted_index.Median | 142 |
| abstract_inverted_index.animal | 273 |
| abstract_inverted_index.assess | 26, 276 |
| abstract_inverted_index.count. | 314 |
| abstract_inverted_index.doses) | 85 |
| abstract_inverted_index.group) | 57 |
| abstract_inverted_index.group, | 93, 101, 133, 181 |
| abstract_inverted_index.groups | 226 |
| abstract_inverted_index.median | 117, 159 |
| abstract_inverted_index.models | 274 |
| abstract_inverted_index.number | 197 |
| abstract_inverted_index.sample | 265 |
| abstract_inverted_index.score, | 308 |
| abstract_inverted_index.scores | 145 |
| abstract_inverted_index.showed | 146 |
| abstract_inverted_index.slower | 15 |
| abstract_inverted_index.titers | 223 |
| abstract_inverted_index.toward | 294 |
| abstract_inverted_index.weeks, | 128 |
| abstract_inverted_index.weeks. | 76 |
| abstract_inverted_index.0.001). | 214 |
| abstract_inverted_index.0.125). | 254 |
| abstract_inverted_index.applied | 68 |
| abstract_inverted_index.calcium | 53, 61 |
| abstract_inverted_index.earlier | 148, 295, 303 |
| abstract_inverted_index.effects | 257 |
| abstract_inverted_index.greater | 196, 310 |
| abstract_inverted_index.group), | 67 |
| abstract_inverted_index.groups, | 78 |
| abstract_inverted_index.mucosal | 208 |
| abstract_inverted_index.nanogel | 281 |
| abstract_inverted_index.placebo | 100, 132, 180, 249 |
| abstract_inverted_index.reduced | 164 |
| abstract_inverted_index.studies | 271 |
| abstract_inverted_index.topical | 31, 91, 123, 167, 202, 241, 285 |
| abstract_inverted_index.(placebo | 66 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.Methods: | 24 |
| abstract_inverted_index.Patients | 22 |
| abstract_inverted_index.Results: | 88 |
| abstract_inverted_index.achieved | 105 |
| abstract_inverted_index.adjuvant | 30 |
| abstract_inverted_index.alginate | 54, 62 |
| abstract_inverted_index.compared | 18 |
| abstract_inverted_index.efficacy | 28 |
| abstract_inverted_index.erosions | 73 |
| abstract_inverted_index.lesions. | 21 |
| abstract_inverted_index.nanogel, | 289 |
| abstract_inverted_index.negative | 207 |
| abstract_inverted_index.painful, | 9 |
| abstract_inverted_index.patients | 44, 96, 104 |
| abstract_inverted_index.presents | 6 |
| abstract_inverted_index.received | 81 |
| abstract_inverted_index.response | 17 |
| abstract_inverted_index.therapy. | 115 |
| abstract_inverted_index.vulgaris | 4 |
| abstract_inverted_index.(tapering | 84 |
| abstract_inverted_index.addition, | 80 |
| abstract_inverted_index.cutaneous | 20 |
| abstract_inverted_index.erosions, | 12 |
| abstract_inverted_index.increased | 184 |
| abstract_inverted_index.observed. | 261 |
| abstract_inverted_index.pemphigus | 3, 144, 307 |
| abstract_inverted_index.reduction | 149, 311 |
| abstract_inverted_index.remission | 106, 120 |
| abstract_inverted_index.rituximab | 32, 50, 92, 124, 152, 168, 203, 242, 259, 279, 286 |
| abstract_inverted_index.treatment | 16 |
| abstract_inverted_index.absorption | 277 |
| abstract_inverted_index.comparable | 218 |
| abstract_inverted_index.cumulative | 233, 299 |
| abstract_inverted_index.desmoglein | 221 |
| abstract_inverted_index.nonhealing | 10 |
| abstract_inverted_index.randomized | 48 |
| abstract_inverted_index.recruited, | 45 |
| abstract_inverted_index.remission, | 296 |
| abstract_inverted_index.(intergroup | 190 |
| abstract_inverted_index.Background: | 1 |
| abstract_inverted_index.Conclusion: | 283 |
| abstract_inverted_index.circulating | 160 |
| abstract_inverted_index.improvement | 304 |
| abstract_inverted_index.persistent, | 8 |
| abstract_inverted_index.Limitations: | 262 |
| abstract_inverted_index.encapsulated | 33 |
| abstract_inverted_index.formulation. | 282 |
| abstract_inverted_index.incorporated | 51, 287 |
| abstract_inverted_index.nanoparticle | 35, 63 |
| abstract_inverted_index.prednisolone | 83, 234, 301 |
| abstract_inverted_index.azathioprine. | 87 |
| abstract_inverted_index.nanoparticles | 55 |
| abstract_inverted_index.significantly | 195 |
| abstract_inverted_index.pharmacokinetic | 270 |
| cited_by_percentile_year.max | 95 |
| cited_by_percentile_year.min | 91 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 7 |
| citation_normalized_percentile.value | 0.88646237 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | True |