CTNI-42. NAVIGATED FOCUSED ULTRASOUND COMBINED WITH RADIOTHERAPY FOR MALIGNANT GLIOMA: A PILOT CLINICAL STUDY Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1093/neuonc/noaf201.0538
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1093/neuonc/noaf201.0538
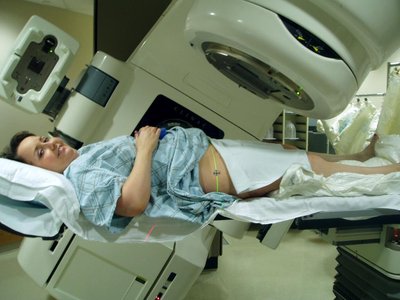
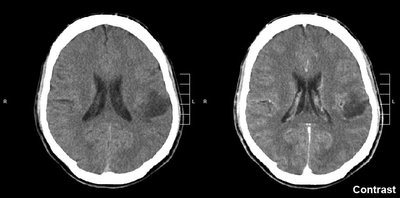
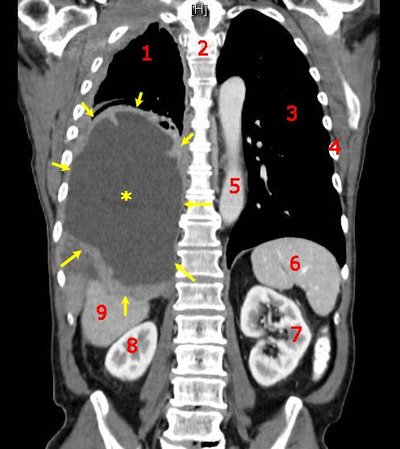
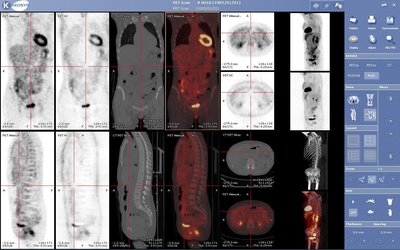
INTRODUCTION Blood–brain barrier (BBB) remains to be the major obstacle to conquer in treating patients with malignant glioma. Hypoxic tumor microenvironment impaired the effect of radiation induced cell death by Radiation therapy (RT). Focused ultrasound (FUS) combined with systemic microbubbles has been shown not only to open BBB but also potentially increased regional perfusion in preclinical study. However, no clinical study has investigated the combination of RT with FUS-BBB opening (RT-FUS). METHODS We aimed to report a 1-yr analysis of a single arm, prospective, pilot study (NCT04988750) of combining RT-FUS for recurrent high grade glioma patients, of whom re-RT was considered for disease focal control. In both preclinical and clinical studies, FUS-BBB opening protocol was conducted within 2 h before RT. Treatment responses were evaluated by objective response rate (ORR) using magnetic resonance imaging, progression free survival, and overall survival, and adverse events (AE) in clinical study. RESULTS In the pilot clinical trial, enrolled 7 recurrent high grade glioma patients who underwent a total of 24 RT-FUS treatments was presented. Three patients had rapid disease progression at a mean of 33 days after RT-FUS, while another three patients had at least stable disease (mean 323 days) after RT-FUS with or without salvage chemotherapy or target therapy. One patient had partial response after RT-FUS, making the ORR of 16.7%. There was no FUS-related AEs, but one (16.7%) re-RT-related grade three radiation necrosis. CONCLUSION Reirradiation is becoming an option after disease recurrence for both primary and secondary malignant brain tumors since systemic therapy significantly prolongs survival in cancer patients. The mechanism behind the synergistic effect of RT-FUS in preclinical model needs further study. The clinical evidence from the pilot clinical trial (NCT04988750) showed a combination of RT-FUS was safe (no FUS-related adverse effect) and a possible synergic effect.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1093/neuonc/noaf201.0538
- https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdf
- OA Status
- bronze
- OpenAlex ID
- https://openalex.org/W7104740301
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W7104740301Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1093/neuonc/noaf201.0538Digital Object Identifier
- Title
-
CTNI-42. NAVIGATED FOCUSED ULTRASOUND COMBINED WITH RADIOTHERAPY FOR MALIGNANT GLIOMA: A PILOT CLINICAL STUDYWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-11-01Full publication date if available
- Authors
-
Ya-Jui Lin, Ko-Ting Chn, Chiung-Yin Huang, Kuo Chen WeiList of authors in order
- Landing page
-
https://doi.org/10.1093/neuonc/noaf201.0538Publisher landing page
- PDF URL
-
https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
bronzeOpen access status per OpenAlex
- OA URL
-
https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdfDirect OA link when available
- Concepts
-
Medicine, Radiation therapy, Clinical study, Radiology, Progressive disease, Clinical trial, Magnetic resonance imaging, Chemotherapy, Adverse effect, Glioma, Focused ultrasound, Disease, Ultrasound, Microbubbles, Complete response, Oncology, Surgery, Stage (stratigraphy), Tumor progression, Phases of clinical research, Perfusion, Nuclear medicine, High-intensity focused ultrasound, Internal medicine, Clinical researchTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
Full payload
| id | https://openalex.org/W7104740301 |
|---|---|
| doi | https://doi.org/10.1093/neuonc/noaf201.0538 |
| ids.doi | https://doi.org/10.1093/neuonc/noaf201.0538 |
| ids.openalex | https://openalex.org/W7104740301 |
| fwci | 0.0 |
| type | article |
| title | CTNI-42. NAVIGATED FOCUSED ULTRASOUND COMBINED WITH RADIOTHERAPY FOR MALIGNANT GLIOMA: A PILOT CLINICAL STUDY |
| biblio.issue | Supplement_5 |
| biblio.volume | 27 |
| biblio.last_page | v136 |
| biblio.first_page | v135 |
| topics[0].id | https://openalex.org/T10958 |
| topics[0].field.id | https://openalex.org/fields/22 |
| topics[0].field.display_name | Engineering |
| topics[0].score | 0.892585039138794 |
| topics[0].domain.id | https://openalex.org/domains/3 |
| topics[0].domain.display_name | Physical Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2204 |
| topics[0].subfield.display_name | Biomedical Engineering |
| topics[0].display_name | Ultrasound and Hyperthermia Applications |
| topics[1].id | https://openalex.org/T10129 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.02124486304819584 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2716 |
| topics[1].subfield.display_name | Genetics |
| topics[1].display_name | Glioma Diagnosis and Treatment |
| topics[2].id | https://openalex.org/T11359 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.015881787985563278 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2741 |
| topics[2].subfield.display_name | Radiology, Nuclear Medicine and Imaging |
| topics[2].display_name | Effects of Radiation Exposure |
| is_xpac | False |
| apc_list.value | 4612 |
| apc_list.currency | USD |
| apc_list.value_usd | 4612 |
| apc_paid | |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.928959846496582 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C509974204 |
| concepts[1].level | 2 |
| concepts[1].score | 0.617897093296051 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q180507 |
| concepts[1].display_name | Radiation therapy |
| concepts[2].id | https://openalex.org/C2987828669 |
| concepts[2].level | 2 |
| concepts[2].score | 0.5500030517578125 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[2].display_name | Clinical study |
| concepts[3].id | https://openalex.org/C126838900 |
| concepts[3].level | 1 |
| concepts[3].score | 0.4991987943649292 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q77604 |
| concepts[3].display_name | Radiology |
| concepts[4].id | https://openalex.org/C2778822529 |
| concepts[4].level | 3 |
| concepts[4].score | 0.47760507464408875 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q1951525 |
| concepts[4].display_name | Progressive disease |
| concepts[5].id | https://openalex.org/C535046627 |
| concepts[5].level | 2 |
| concepts[5].score | 0.4597589671611786 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[5].display_name | Clinical trial |
| concepts[6].id | https://openalex.org/C143409427 |
| concepts[6].level | 2 |
| concepts[6].score | 0.42737510800361633 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q161238 |
| concepts[6].display_name | Magnetic resonance imaging |
| concepts[7].id | https://openalex.org/C2776694085 |
| concepts[7].level | 2 |
| concepts[7].score | 0.4044847786426544 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q974135 |
| concepts[7].display_name | Chemotherapy |
| concepts[8].id | https://openalex.org/C197934379 |
| concepts[8].level | 2 |
| concepts[8].score | 0.3991687595844269 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[8].display_name | Adverse effect |
| concepts[9].id | https://openalex.org/C2778227246 |
| concepts[9].level | 2 |
| concepts[9].score | 0.3682345449924469 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q1365309 |
| concepts[9].display_name | Glioma |
| concepts[10].id | https://openalex.org/C2985948822 |
| concepts[10].level | 3 |
| concepts[10].score | 0.3543170690536499 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q564897 |
| concepts[10].display_name | Focused ultrasound |
| concepts[11].id | https://openalex.org/C2779134260 |
| concepts[11].level | 2 |
| concepts[11].score | 0.3437695801258087 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q12136 |
| concepts[11].display_name | Disease |
| concepts[12].id | https://openalex.org/C143753070 |
| concepts[12].level | 2 |
| concepts[12].score | 0.3256472647190094 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q162564 |
| concepts[12].display_name | Ultrasound |
| concepts[13].id | https://openalex.org/C64012434 |
| concepts[13].level | 3 |
| concepts[13].score | 0.3125837743282318 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q6839290 |
| concepts[13].display_name | Microbubbles |
| concepts[14].id | https://openalex.org/C3019096185 |
| concepts[14].level | 3 |
| concepts[14].score | 0.31244373321533203 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q1340863 |
| concepts[14].display_name | Complete response |
| concepts[15].id | https://openalex.org/C143998085 |
| concepts[15].level | 1 |
| concepts[15].score | 0.3034321963787079 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q162555 |
| concepts[15].display_name | Oncology |
| concepts[16].id | https://openalex.org/C141071460 |
| concepts[16].level | 1 |
| concepts[16].score | 0.2974156141281128 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q40821 |
| concepts[16].display_name | Surgery |
| concepts[17].id | https://openalex.org/C146357865 |
| concepts[17].level | 2 |
| concepts[17].score | 0.29499340057373047 |
| concepts[17].wikidata | https://www.wikidata.org/wiki/Q1123245 |
| concepts[17].display_name | Stage (stratigraphy) |
| concepts[18].id | https://openalex.org/C2779256057 |
| concepts[18].level | 3 |
| concepts[18].score | 0.2859238386154175 |
| concepts[18].wikidata | https://www.wikidata.org/wiki/Q16909647 |
| concepts[18].display_name | Tumor progression |
| concepts[19].id | https://openalex.org/C31760486 |
| concepts[19].level | 3 |
| concepts[19].score | 0.2859037220478058 |
| concepts[19].wikidata | https://www.wikidata.org/wiki/Q7180990 |
| concepts[19].display_name | Phases of clinical research |
| concepts[20].id | https://openalex.org/C146957229 |
| concepts[20].level | 2 |
| concepts[20].score | 0.27052438259124756 |
| concepts[20].wikidata | https://www.wikidata.org/wiki/Q1266915 |
| concepts[20].display_name | Perfusion |
| concepts[21].id | https://openalex.org/C2989005 |
| concepts[21].level | 1 |
| concepts[21].score | 0.2628417909145355 |
| concepts[21].wikidata | https://www.wikidata.org/wiki/Q214963 |
| concepts[21].display_name | Nuclear medicine |
| concepts[22].id | https://openalex.org/C2777365067 |
| concepts[22].level | 3 |
| concepts[22].score | 0.2625168561935425 |
| concepts[22].wikidata | https://www.wikidata.org/wiki/Q564897 |
| concepts[22].display_name | High-intensity focused ultrasound |
| concepts[23].id | https://openalex.org/C126322002 |
| concepts[23].level | 1 |
| concepts[23].score | 0.253677099943161 |
| concepts[23].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[23].display_name | Internal medicine |
| concepts[24].id | https://openalex.org/C502991105 |
| concepts[24].level | 2 |
| concepts[24].score | 0.25162315368652344 |
| concepts[24].wikidata | https://www.wikidata.org/wiki/Q5133849 |
| concepts[24].display_name | Clinical research |
| keywords[0].id | https://openalex.org/keywords/radiation-therapy |
| keywords[0].score | 0.617897093296051 |
| keywords[0].display_name | Radiation therapy |
| keywords[1].id | https://openalex.org/keywords/clinical-study |
| keywords[1].score | 0.5500030517578125 |
| keywords[1].display_name | Clinical study |
| keywords[2].id | https://openalex.org/keywords/progressive-disease |
| keywords[2].score | 0.47760507464408875 |
| keywords[2].display_name | Progressive disease |
| keywords[3].id | https://openalex.org/keywords/clinical-trial |
| keywords[3].score | 0.4597589671611786 |
| keywords[3].display_name | Clinical trial |
| keywords[4].id | https://openalex.org/keywords/magnetic-resonance-imaging |
| keywords[4].score | 0.42737510800361633 |
| keywords[4].display_name | Magnetic resonance imaging |
| keywords[5].id | https://openalex.org/keywords/chemotherapy |
| keywords[5].score | 0.4044847786426544 |
| keywords[5].display_name | Chemotherapy |
| keywords[6].id | https://openalex.org/keywords/adverse-effect |
| keywords[6].score | 0.3991687595844269 |
| keywords[6].display_name | Adverse effect |
| keywords[7].id | https://openalex.org/keywords/glioma |
| keywords[7].score | 0.3682345449924469 |
| keywords[7].display_name | Glioma |
| language | en |
| locations[0].id | doi:10.1093/neuonc/noaf201.0538 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S106908163 |
| locations[0].source.issn | 1522-8517, 1523-5866 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1522-8517 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Neuro-Oncology |
| locations[0].source.host_organization | https://openalex.org/P4310311648 |
| locations[0].source.host_organization_name | Oxford University Press |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310311648 |
| locations[0].license | |
| locations[0].pdf_url | https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Neuro-Oncology |
| locations[0].landing_page_url | https://doi.org/10.1093/neuonc/noaf201.0538 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A2707275213 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-4339-0147 |
| authorships[0].author.display_name | Ya-Jui Lin |
| authorships[0].affiliations[0].raw_affiliation_string | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Ya-Jui Lin |
| authorships[0].is_corresponding | True |
| authorships[0].raw_affiliation_strings | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[1].author.id | |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Ko-Ting Chn |
| authorships[1].affiliations[0].raw_affiliation_string | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Ko-Ting Chn |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[2].author.id | https://openalex.org/A2584644843 |
| authorships[2].author.orcid | https://orcid.org/0000-0001-6954-9737 |
| authorships[2].author.display_name | Chiung-Yin Huang |
| authorships[2].affiliations[0].raw_affiliation_string | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Chiung-Yin Huang |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[3].author.id | https://openalex.org/A2024247755 |
| authorships[3].author.orcid | https://orcid.org/0000-0003-3505-2196 |
| authorships[3].author.display_name | Kuo Chen Wei |
| authorships[3].affiliations[0].raw_affiliation_string | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| authorships[3].author_position | last |
| authorships[3].raw_author_name | Kuo-Chen Wei |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Chang-Gung memorial hospital , Taoyuan, Taiwan |
| has_content.pdf | True |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdf |
| open_access.oa_status | bronze |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-11-11T00:00:00 |
| display_name | CTNI-42. NAVIGATED FOCUSED ULTRASOUND COMBINED WITH RADIOTHERAPY FOR MALIGNANT GLIOMA: A PILOT CLINICAL STUDY |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-11T23:23:10.385787 |
| primary_topic.id | https://openalex.org/T10958 |
| primary_topic.field.id | https://openalex.org/fields/22 |
| primary_topic.field.display_name | Engineering |
| primary_topic.score | 0.892585039138794 |
| primary_topic.domain.id | https://openalex.org/domains/3 |
| primary_topic.domain.display_name | Physical Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2204 |
| primary_topic.subfield.display_name | Biomedical Engineering |
| primary_topic.display_name | Ultrasound and Hyperthermia Applications |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | doi:10.1093/neuonc/noaf201.0538 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S106908163 |
| best_oa_location.source.issn | 1522-8517, 1523-5866 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | 1522-8517 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | Neuro-Oncology |
| best_oa_location.source.host_organization | https://openalex.org/P4310311648 |
| best_oa_location.source.host_organization_name | Oxford University Press |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310311648 |
| best_oa_location.license | |
| best_oa_location.pdf_url | https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Neuro-Oncology |
| best_oa_location.landing_page_url | https://doi.org/10.1093/neuonc/noaf201.0538 |
| primary_location.id | doi:10.1093/neuonc/noaf201.0538 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S106908163 |
| primary_location.source.issn | 1522-8517, 1523-5866 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1522-8517 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Neuro-Oncology |
| primary_location.source.host_organization | https://openalex.org/P4310311648 |
| primary_location.source.host_organization_name | Oxford University Press |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310311648 |
| primary_location.license | |
| primary_location.pdf_url | https://academic.oup.com/neuro-oncology/article-pdf/27/Supplement_5/v135/65255230/noaf201.0538.pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Neuro-Oncology |
| primary_location.landing_page_url | https://doi.org/10.1093/neuonc/noaf201.0538 |
| publication_date | 2025-11-01 |
| publication_year | 2025 |
| referenced_works_count | 0 |
| abstract_inverted_index.2 | 118 |
| abstract_inverted_index.7 | 155 |
| abstract_inverted_index.a | 77, 81, 163, 178, 282, 293 |
| abstract_inverted_index.h | 119 |
| abstract_inverted_index.24 | 166 |
| abstract_inverted_index.33 | 181 |
| abstract_inverted_index.In | 106, 149 |
| abstract_inverted_index.RT | 67 |
| abstract_inverted_index.We | 73 |
| abstract_inverted_index.an | 236 |
| abstract_inverted_index.at | 177, 190 |
| abstract_inverted_index.be | 7 |
| abstract_inverted_index.by | 30, 126 |
| abstract_inverted_index.in | 13, 55, 145, 255, 266 |
| abstract_inverted_index.is | 234 |
| abstract_inverted_index.no | 59, 221 |
| abstract_inverted_index.of | 25, 66, 80, 88, 97, 165, 180, 217, 264, 284 |
| abstract_inverted_index.or | 200, 204 |
| abstract_inverted_index.to | 6, 11, 46, 75 |
| abstract_inverted_index.(no | 288 |
| abstract_inverted_index.323 | 195 |
| abstract_inverted_index.BBB | 48 |
| abstract_inverted_index.ORR | 216 |
| abstract_inverted_index.One | 207 |
| abstract_inverted_index.RT. | 121 |
| abstract_inverted_index.The | 258, 272 |
| abstract_inverted_index.and | 109, 138, 141, 244, 292 |
| abstract_inverted_index.but | 49, 224 |
| abstract_inverted_index.for | 91, 102, 241 |
| abstract_inverted_index.had | 173, 189, 209 |
| abstract_inverted_index.has | 41, 62 |
| abstract_inverted_index.not | 44 |
| abstract_inverted_index.one | 225 |
| abstract_inverted_index.the | 8, 23, 64, 150, 215, 261, 276 |
| abstract_inverted_index.was | 100, 115, 169, 220, 286 |
| abstract_inverted_index.who | 161 |
| abstract_inverted_index.(AE) | 144 |
| abstract_inverted_index.1-yr | 78 |
| abstract_inverted_index.AEs, | 223 |
| abstract_inverted_index.also | 50 |
| abstract_inverted_index.arm, | 83 |
| abstract_inverted_index.been | 42 |
| abstract_inverted_index.both | 107, 242 |
| abstract_inverted_index.cell | 28 |
| abstract_inverted_index.days | 182 |
| abstract_inverted_index.free | 136 |
| abstract_inverted_index.from | 275 |
| abstract_inverted_index.high | 93, 157 |
| abstract_inverted_index.mean | 179 |
| abstract_inverted_index.only | 45 |
| abstract_inverted_index.open | 47 |
| abstract_inverted_index.rate | 129 |
| abstract_inverted_index.safe | 287 |
| abstract_inverted_index.were | 124 |
| abstract_inverted_index.whom | 98 |
| abstract_inverted_index.with | 16, 38, 68, 199 |
| abstract_inverted_index.(BBB) | 4 |
| abstract_inverted_index.(FUS) | 36 |
| abstract_inverted_index.(ORR) | 130 |
| abstract_inverted_index.(RT). | 33 |
| abstract_inverted_index.(mean | 194 |
| abstract_inverted_index.There | 219 |
| abstract_inverted_index.Three | 171 |
| abstract_inverted_index.after | 183, 197, 212, 238 |
| abstract_inverted_index.aimed | 74 |
| abstract_inverted_index.brain | 247 |
| abstract_inverted_index.days) | 196 |
| abstract_inverted_index.death | 29 |
| abstract_inverted_index.focal | 104 |
| abstract_inverted_index.grade | 94, 158, 228 |
| abstract_inverted_index.least | 191 |
| abstract_inverted_index.major | 9 |
| abstract_inverted_index.model | 268 |
| abstract_inverted_index.needs | 269 |
| abstract_inverted_index.pilot | 85, 151, 277 |
| abstract_inverted_index.rapid | 174 |
| abstract_inverted_index.re-RT | 99 |
| abstract_inverted_index.shown | 43 |
| abstract_inverted_index.since | 249 |
| abstract_inverted_index.study | 61, 86 |
| abstract_inverted_index.three | 187, 229 |
| abstract_inverted_index.total | 164 |
| abstract_inverted_index.trial | 279 |
| abstract_inverted_index.tumor | 20 |
| abstract_inverted_index.using | 131 |
| abstract_inverted_index.while | 185 |
| abstract_inverted_index.16.7%. | 218 |
| abstract_inverted_index.RT-FUS | 90, 167, 198, 265, 285 |
| abstract_inverted_index.before | 120 |
| abstract_inverted_index.behind | 260 |
| abstract_inverted_index.cancer | 256 |
| abstract_inverted_index.effect | 24, 263 |
| abstract_inverted_index.events | 143 |
| abstract_inverted_index.glioma | 95, 159 |
| abstract_inverted_index.making | 214 |
| abstract_inverted_index.option | 237 |
| abstract_inverted_index.report | 76 |
| abstract_inverted_index.showed | 281 |
| abstract_inverted_index.single | 82 |
| abstract_inverted_index.stable | 192 |
| abstract_inverted_index.study. | 57, 147, 271 |
| abstract_inverted_index.target | 205 |
| abstract_inverted_index.trial, | 153 |
| abstract_inverted_index.tumors | 248 |
| abstract_inverted_index.within | 117 |
| abstract_inverted_index.(16.7%) | 226 |
| abstract_inverted_index.FUS-BBB | 69, 112 |
| abstract_inverted_index.Focused | 34 |
| abstract_inverted_index.Hypoxic | 19 |
| abstract_inverted_index.METHODS | 72 |
| abstract_inverted_index.RESULTS | 148 |
| abstract_inverted_index.RT-FUS, | 184, 213 |
| abstract_inverted_index.adverse | 142, 290 |
| abstract_inverted_index.another | 186 |
| abstract_inverted_index.barrier | 3 |
| abstract_inverted_index.conquer | 12 |
| abstract_inverted_index.disease | 103, 175, 193, 239 |
| abstract_inverted_index.effect) | 291 |
| abstract_inverted_index.effect. | 296 |
| abstract_inverted_index.further | 270 |
| abstract_inverted_index.glioma. | 18 |
| abstract_inverted_index.induced | 27 |
| abstract_inverted_index.opening | 70, 113 |
| abstract_inverted_index.overall | 139 |
| abstract_inverted_index.partial | 210 |
| abstract_inverted_index.patient | 208 |
| abstract_inverted_index.primary | 243 |
| abstract_inverted_index.remains | 5 |
| abstract_inverted_index.salvage | 202 |
| abstract_inverted_index.therapy | 32, 251 |
| abstract_inverted_index.without | 201 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.However, | 58 |
| abstract_inverted_index.analysis | 79 |
| abstract_inverted_index.becoming | 235 |
| abstract_inverted_index.clinical | 60, 110, 146, 152, 273, 278 |
| abstract_inverted_index.combined | 37 |
| abstract_inverted_index.control. | 105 |
| abstract_inverted_index.enrolled | 154 |
| abstract_inverted_index.evidence | 274 |
| abstract_inverted_index.imaging, | 134 |
| abstract_inverted_index.impaired | 22 |
| abstract_inverted_index.magnetic | 132 |
| abstract_inverted_index.obstacle | 10 |
| abstract_inverted_index.patients | 15, 160, 172, 188 |
| abstract_inverted_index.possible | 294 |
| abstract_inverted_index.prolongs | 253 |
| abstract_inverted_index.protocol | 114 |
| abstract_inverted_index.regional | 53 |
| abstract_inverted_index.response | 128, 211 |
| abstract_inverted_index.studies, | 111 |
| abstract_inverted_index.survival | 254 |
| abstract_inverted_index.synergic | 295 |
| abstract_inverted_index.systemic | 39, 250 |
| abstract_inverted_index.therapy. | 206 |
| abstract_inverted_index.treating | 14 |
| abstract_inverted_index.(RT-FUS). | 71 |
| abstract_inverted_index.Radiation | 31 |
| abstract_inverted_index.Treatment | 122 |
| abstract_inverted_index.combining | 89 |
| abstract_inverted_index.conducted | 116 |
| abstract_inverted_index.evaluated | 125 |
| abstract_inverted_index.increased | 52 |
| abstract_inverted_index.malignant | 17, 246 |
| abstract_inverted_index.mechanism | 259 |
| abstract_inverted_index.necrosis. | 231 |
| abstract_inverted_index.objective | 127 |
| abstract_inverted_index.patients, | 96 |
| abstract_inverted_index.patients. | 257 |
| abstract_inverted_index.perfusion | 54 |
| abstract_inverted_index.radiation | 26, 230 |
| abstract_inverted_index.recurrent | 92, 156 |
| abstract_inverted_index.resonance | 133 |
| abstract_inverted_index.responses | 123 |
| abstract_inverted_index.secondary | 245 |
| abstract_inverted_index.survival, | 137, 140 |
| abstract_inverted_index.underwent | 162 |
| abstract_inverted_index.CONCLUSION | 232 |
| abstract_inverted_index.considered | 101 |
| abstract_inverted_index.presented. | 170 |
| abstract_inverted_index.recurrence | 240 |
| abstract_inverted_index.treatments | 168 |
| abstract_inverted_index.ultrasound | 35 |
| abstract_inverted_index.FUS-related | 222, 289 |
| abstract_inverted_index.combination | 65, 283 |
| abstract_inverted_index.potentially | 51 |
| abstract_inverted_index.preclinical | 56, 108, 267 |
| abstract_inverted_index.progression | 135, 176 |
| abstract_inverted_index.synergistic | 262 |
| abstract_inverted_index.INTRODUCTION | 1 |
| abstract_inverted_index.chemotherapy | 203 |
| abstract_inverted_index.investigated | 63 |
| abstract_inverted_index.microbubbles | 40 |
| abstract_inverted_index.prospective, | 84 |
| abstract_inverted_index.(NCT04988750) | 87, 280 |
| abstract_inverted_index.Blood–brain | 2 |
| abstract_inverted_index.Reirradiation | 233 |
| abstract_inverted_index.re-RT-related | 227 |
| abstract_inverted_index.significantly | 252 |
| abstract_inverted_index.microenvironment | 21 |
| cited_by_percentile_year | |
| countries_distinct_count | 0 |
| institutions_distinct_count | 4 |
| citation_normalized_percentile.value | 0.70657288 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |