Development, optimization, and in-vivo bioavailability study of erlotinib hydrochloride loaded microsponge for colon targeting Article Swipe
 YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1016/j.ipha.2024.07.002
YOU?
·
· 2024
· Open Access
·
· DOI: https://doi.org/10.1016/j.ipha.2024.07.002
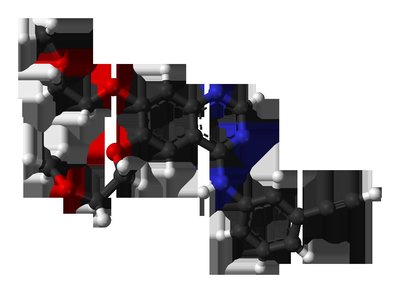
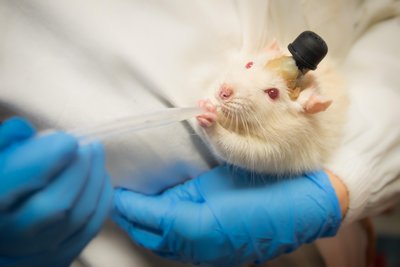
The present investigation aimed to develop as well as optimize microsponge containing erlotinib hydrochloride (ETB) that was composed of ethyl cellulose (EC) and pectin. The water solubility and enzymatic susceptibility make it easier to fabricate the microsponge formulation. The ETB loaded microsponge was manufactured using quasi-emulsion solvent diffusion process. By this technique, organic solution of the primary component is emulsified with stabilizing agents that are soluble in water. To design the formation of the microsponge, 32 factorial design was implemented. It was investigated how the response variables like particle dimension, entrapment efficiency, ETB diffusion at 12 h were influenced by independent variables such as rotation speed and the pectin to ethyl cellulose ratio. The optimal microsponge formulation loaded with ETB (F0) composed of 1:2.8 ratio of pectin to ethyl cellulose (EC) with stirring rate at 478 rpm. Particle dimension, entrapment efficiency, and ETB release at 12 h from optimized formulation were shown 104.89 ± 0.62 nm, 82.36 ± 2.85 %, and 85.49 ± 1.84 % respectively. The In-vivo pharmacokinetic study conducted on rabbit model shows a significant improvement in bioavailability. The optimized microsponge formulation has been found to have a higher Cmax than the ETB aqueous suspension. The stability of the formulation has been determined by the accelerated stability study of optimized microsponge formulation. This study indicated that the optimized formulation retained its stability even after 90days. In general, the present investigation demonstrated that drug loaded microsponge based formulation is a suitable method to improve the therapeutic efficacy and bioavailability of ETB.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1016/j.ipha.2024.07.002
- OA Status
- diamond
- Cited By
- 3
- References
- 42
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4400308803
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4400308803Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1016/j.ipha.2024.07.002Digital Object Identifier
- Title
-
Development, optimization, and in-vivo bioavailability study of erlotinib hydrochloride loaded microsponge for colon targetingWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2024Year of publication
- Publication date
-
2024-07-03Full publication date if available
- Authors
-
Ayan Kar, Beduin Mahanti, Banhishikha Kar, Anupam Jana, Subhasis Chakrabarty, Smriti Singh, Subhabrota MajumdarList of authors in order
- Landing page
-
https://doi.org/10.1016/j.ipha.2024.07.002Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
diamondOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.1016/j.ipha.2024.07.002Direct OA link when available
- Concepts
-
Bioavailability, Erlotinib, In vivo, Pharmacology, Biosimilar, Medicine, Hydrochloride, Erlotinib Hydrochloride, Chemistry, Internal medicine, Cancer, Biotechnology, Epidermal growth factor receptor, Organic chemistry, BiologyTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
3Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 2, 2024: 1Per-year citation counts (last 5 years)
- References (count)
-
42Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4400308803 |
|---|---|
| doi | https://doi.org/10.1016/j.ipha.2024.07.002 |
| ids.doi | https://doi.org/10.1016/j.ipha.2024.07.002 |
| ids.openalex | https://openalex.org/W4400308803 |
| fwci | 2.58786942 |
| type | article |
| title | Development, optimization, and in-vivo bioavailability study of erlotinib hydrochloride loaded microsponge for colon targeting |
| biblio.issue | 1 |
| biblio.volume | 3 |
| biblio.last_page | 10 |
| biblio.first_page | 1 |
| topics[0].id | https://openalex.org/T10696 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9968000054359436 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2740 |
| topics[0].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[0].display_name | Gastric Cancer Management and Outcomes |
| topics[1].id | https://openalex.org/T10256 |
| topics[1].field.id | https://openalex.org/fields/30 |
| topics[1].field.display_name | Pharmacology, Toxicology and Pharmaceutics |
| topics[1].score | 0.9961000084877014 |
| topics[1].domain.id | https://openalex.org/domains/1 |
| topics[1].domain.display_name | Life Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/3003 |
| topics[1].subfield.display_name | Pharmaceutical Science |
| topics[1].display_name | Drug Solubulity and Delivery Systems |
| topics[2].id | https://openalex.org/T10231 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9904999732971191 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2730 |
| topics[2].subfield.display_name | Oncology |
| topics[2].display_name | Pancreatic and Hepatic Oncology Research |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C181389837 |
| concepts[0].level | 2 |
| concepts[0].score | 0.870686948299408 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q461809 |
| concepts[0].display_name | Bioavailability |
| concepts[1].id | https://openalex.org/C2778087573 |
| concepts[1].level | 4 |
| concepts[1].score | 0.722674548625946 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q418369 |
| concepts[1].display_name | Erlotinib |
| concepts[2].id | https://openalex.org/C207001950 |
| concepts[2].level | 2 |
| concepts[2].score | 0.7089784145355225 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q141124 |
| concepts[2].display_name | In vivo |
| concepts[3].id | https://openalex.org/C98274493 |
| concepts[3].level | 1 |
| concepts[3].score | 0.5961684584617615 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q128406 |
| concepts[3].display_name | Pharmacology |
| concepts[4].id | https://openalex.org/C59491497 |
| concepts[4].level | 2 |
| concepts[4].score | 0.5592002272605896 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q864715 |
| concepts[4].display_name | Biosimilar |
| concepts[5].id | https://openalex.org/C71924100 |
| concepts[5].level | 0 |
| concepts[5].score | 0.4585120677947998 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[5].display_name | Medicine |
| concepts[6].id | https://openalex.org/C2776460385 |
| concepts[6].level | 2 |
| concepts[6].score | 0.4288502037525177 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q420060 |
| concepts[6].display_name | Hydrochloride |
| concepts[7].id | https://openalex.org/C2909325608 |
| concepts[7].level | 5 |
| concepts[7].score | 0.4282803535461426 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q418369 |
| concepts[7].display_name | Erlotinib Hydrochloride |
| concepts[8].id | https://openalex.org/C185592680 |
| concepts[8].level | 0 |
| concepts[8].score | 0.254041850566864 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q2329 |
| concepts[8].display_name | Chemistry |
| concepts[9].id | https://openalex.org/C126322002 |
| concepts[9].level | 1 |
| concepts[9].score | 0.18926340341567993 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[9].display_name | Internal medicine |
| concepts[10].id | https://openalex.org/C121608353 |
| concepts[10].level | 2 |
| concepts[10].score | 0.11786916851997375 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[10].display_name | Cancer |
| concepts[11].id | https://openalex.org/C150903083 |
| concepts[11].level | 1 |
| concepts[11].score | 0.07167938351631165 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q7108 |
| concepts[11].display_name | Biotechnology |
| concepts[12].id | https://openalex.org/C2779438470 |
| concepts[12].level | 3 |
| concepts[12].score | 0.06325370073318481 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q424401 |
| concepts[12].display_name | Epidermal growth factor receptor |
| concepts[13].id | https://openalex.org/C178790620 |
| concepts[13].level | 1 |
| concepts[13].score | 0.0 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q11351 |
| concepts[13].display_name | Organic chemistry |
| concepts[14].id | https://openalex.org/C86803240 |
| concepts[14].level | 0 |
| concepts[14].score | 0.0 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q420 |
| concepts[14].display_name | Biology |
| keywords[0].id | https://openalex.org/keywords/bioavailability |
| keywords[0].score | 0.870686948299408 |
| keywords[0].display_name | Bioavailability |
| keywords[1].id | https://openalex.org/keywords/erlotinib |
| keywords[1].score | 0.722674548625946 |
| keywords[1].display_name | Erlotinib |
| keywords[2].id | https://openalex.org/keywords/in-vivo |
| keywords[2].score | 0.7089784145355225 |
| keywords[2].display_name | In vivo |
| keywords[3].id | https://openalex.org/keywords/pharmacology |
| keywords[3].score | 0.5961684584617615 |
| keywords[3].display_name | Pharmacology |
| keywords[4].id | https://openalex.org/keywords/biosimilar |
| keywords[4].score | 0.5592002272605896 |
| keywords[4].display_name | Biosimilar |
| keywords[5].id | https://openalex.org/keywords/medicine |
| keywords[5].score | 0.4585120677947998 |
| keywords[5].display_name | Medicine |
| keywords[6].id | https://openalex.org/keywords/hydrochloride |
| keywords[6].score | 0.4288502037525177 |
| keywords[6].display_name | Hydrochloride |
| keywords[7].id | https://openalex.org/keywords/erlotinib-hydrochloride |
| keywords[7].score | 0.4282803535461426 |
| keywords[7].display_name | Erlotinib Hydrochloride |
| keywords[8].id | https://openalex.org/keywords/chemistry |
| keywords[8].score | 0.254041850566864 |
| keywords[8].display_name | Chemistry |
| keywords[9].id | https://openalex.org/keywords/internal-medicine |
| keywords[9].score | 0.18926340341567993 |
| keywords[9].display_name | Internal medicine |
| keywords[10].id | https://openalex.org/keywords/cancer |
| keywords[10].score | 0.11786916851997375 |
| keywords[10].display_name | Cancer |
| keywords[11].id | https://openalex.org/keywords/biotechnology |
| keywords[11].score | 0.07167938351631165 |
| keywords[11].display_name | Biotechnology |
| keywords[12].id | https://openalex.org/keywords/epidermal-growth-factor-receptor |
| keywords[12].score | 0.06325370073318481 |
| keywords[12].display_name | Epidermal growth factor receptor |
| language | en |
| locations[0].id | doi:10.1016/j.ipha.2024.07.002 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S4387289262 |
| locations[0].source.issn | 2949-866X |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 2949-866X |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Intelligent Pharmacy |
| locations[0].source.host_organization | https://openalex.org/P4310320990 |
| locations[0].source.host_organization_name | Elsevier BV |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320990 |
| locations[0].license | cc-by |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Intelligent Pharmacy |
| locations[0].landing_page_url | https://doi.org/10.1016/j.ipha.2024.07.002 |
| locations[1].id | pmh:oai:doaj.org/article:4365ed26a269492286121ddf22777166 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306401280 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[1].source.host_organization | |
| locations[1].source.host_organization_name | |
| locations[1].source.host_organization_lineage | |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | submittedVersion |
| locations[1].raw_type | article |
| locations[1].license_id | |
| locations[1].is_accepted | False |
| locations[1].is_published | False |
| locations[1].raw_source_name | Intelligent Pharmacy, Vol 3, Iss 1, Pp 1-10 (2025) |
| locations[1].landing_page_url | https://doaj.org/article/4365ed26a269492286121ddf22777166 |
| indexed_in | crossref, doaj |
| authorships[0].author.id | https://openalex.org/A5000031813 |
| authorships[0].author.orcid | https://orcid.org/0000-0001-8144-1032 |
| authorships[0].author.display_name | Ayan Kar |
| authorships[0].countries | IN |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I1288043984 |
| authorships[0].affiliations[0].raw_affiliation_string | School of Pharmacy, Techno India University, EM 4, Sector-V, Kolkata, 700091, West Bengal, India |
| authorships[0].affiliations[1].raw_affiliation_string | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[0].institutions[0].id | https://openalex.org/I1288043984 |
| authorships[0].institutions[0].ror | https://ror.org/00v1y6t69 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I1288043984 |
| authorships[0].institutions[0].country_code | IN |
| authorships[0].institutions[0].display_name | Techno India University |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Ayan Kumar Kar |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India, School of Pharmacy, Techno India University, EM 4, Sector-V, Kolkata, 700091, West Bengal, India |
| authorships[1].author.id | https://openalex.org/A5091764622 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Beduin Mahanti |
| authorships[1].countries | IN |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I1288043984 |
| authorships[1].affiliations[0].raw_affiliation_string | School of Pharmacy, Techno India University, EM 4, Sector-V, Kolkata, 700091, West Bengal, India |
| authorships[1].institutions[0].id | https://openalex.org/I1288043984 |
| authorships[1].institutions[0].ror | https://ror.org/00v1y6t69 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I1288043984 |
| authorships[1].institutions[0].country_code | IN |
| authorships[1].institutions[0].display_name | Techno India University |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Beduin Mahanti |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | School of Pharmacy, Techno India University, EM 4, Sector-V, Kolkata, 700091, West Bengal, India |
| authorships[2].author.id | https://openalex.org/A5080419010 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-2784-1059 |
| authorships[2].author.display_name | Banhishikha Kar |
| authorships[2].affiliations[0].raw_affiliation_string | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Banhishikha Kar |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[3].author.id | https://openalex.org/A5086578748 |
| authorships[3].author.orcid | https://orcid.org/0000-0002-0404-3513 |
| authorships[3].author.display_name | Anupam Jana |
| authorships[3].affiliations[0].raw_affiliation_string | Seacom Pharmacy College, Jaladhulagori, Andul-Mouri, Sankrail, Howrah, 711302, West Bengal, India |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Anupam Jana |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Seacom Pharmacy College, Jaladhulagori, Andul-Mouri, Sankrail, Howrah, 711302, West Bengal, India |
| authorships[4].author.id | https://openalex.org/A5016831782 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Subhasis Chakrabarty |
| authorships[4].affiliations[0].raw_affiliation_string | Department of Pharmaceutics, DmbH Institute of Medical Science, Dadpur, Puinan, Hoogly, 712305, West Bengal, India |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Subhasis Chakrabarty |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Department of Pharmaceutics, DmbH Institute of Medical Science, Dadpur, Puinan, Hoogly, 712305, West Bengal, India |
| authorships[5].author.id | https://openalex.org/A5084909396 |
| authorships[5].author.orcid | |
| authorships[5].author.display_name | Smriti Singh |
| authorships[5].affiliations[0].raw_affiliation_string | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Smriti Singh |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[6].author.id | https://openalex.org/A5078051329 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-1547-1273 |
| authorships[6].author.display_name | Subhabrota Majumdar |
| authorships[6].affiliations[0].raw_affiliation_string | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| authorships[6].author_position | last |
| authorships[6].raw_author_name | Subhabrota Majumdar |
| authorships[6].is_corresponding | True |
| authorships[6].raw_affiliation_strings | Department of Pharmaceutics, Calcutta Institute of Pharmaceutical Technology and AHS, Banitabla, Uluberia, Howrah, 711316, West Bengal, India |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.1016/j.ipha.2024.07.002 |
| open_access.oa_status | diamond |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Development, optimization, and in-vivo bioavailability study of erlotinib hydrochloride loaded microsponge for colon targeting |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10696 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9968000054359436 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2740 |
| primary_topic.subfield.display_name | Pulmonary and Respiratory Medicine |
| primary_topic.display_name | Gastric Cancer Management and Outcomes |
| related_works | https://openalex.org/W4381887247, https://openalex.org/W2150724094, https://openalex.org/W2801862162, https://openalex.org/W2014230197, https://openalex.org/W2120992361, https://openalex.org/W2619172668, https://openalex.org/W2141303400, https://openalex.org/W2104960376, https://openalex.org/W2119956903, https://openalex.org/W3106445303 |
| cited_by_count | 3 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 2 |
| counts_by_year[1].year | 2024 |
| counts_by_year[1].cited_by_count | 1 |
| locations_count | 2 |
| best_oa_location.id | doi:10.1016/j.ipha.2024.07.002 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S4387289262 |
| best_oa_location.source.issn | 2949-866X |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 2949-866X |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Intelligent Pharmacy |
| best_oa_location.source.host_organization | https://openalex.org/P4310320990 |
| best_oa_location.source.host_organization_name | Elsevier BV |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Intelligent Pharmacy |
| best_oa_location.landing_page_url | https://doi.org/10.1016/j.ipha.2024.07.002 |
| primary_location.id | doi:10.1016/j.ipha.2024.07.002 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S4387289262 |
| primary_location.source.issn | 2949-866X |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 2949-866X |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Intelligent Pharmacy |
| primary_location.source.host_organization | https://openalex.org/P4310320990 |
| primary_location.source.host_organization_name | Elsevier BV |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| primary_location.license | cc-by |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Intelligent Pharmacy |
| primary_location.landing_page_url | https://doi.org/10.1016/j.ipha.2024.07.002 |
| publication_date | 2024-07-03 |
| publication_year | 2024 |
| referenced_works | https://openalex.org/W2414365196, https://openalex.org/W2069417660, https://openalex.org/W2114949163, https://openalex.org/W2271070179, https://openalex.org/W2938341668, https://openalex.org/W620044983, https://openalex.org/W6715033525, https://openalex.org/W2131698002, https://openalex.org/W2082926225, https://openalex.org/W1549826455, https://openalex.org/W2003840904, https://openalex.org/W2078116888, https://openalex.org/W1993543020, https://openalex.org/W2013980387, https://openalex.org/W112826108, https://openalex.org/W1965594274, https://openalex.org/W2080013212, https://openalex.org/W4386249491, https://openalex.org/W1966343441, https://openalex.org/W2161544550, https://openalex.org/W3153188565, https://openalex.org/W3200651639, https://openalex.org/W2141260063, https://openalex.org/W6639771040, https://openalex.org/W3101525505, https://openalex.org/W3046847278, https://openalex.org/W2134168039, https://openalex.org/W2103532948, https://openalex.org/W6680593325, https://openalex.org/W6743363716, https://openalex.org/W6733240293, https://openalex.org/W1801010267, https://openalex.org/W6797587414, https://openalex.org/W6628299986, https://openalex.org/W1966894289, https://openalex.org/W4319242164, https://openalex.org/W2588079663, https://openalex.org/W2411444101, https://openalex.org/W3176380193, https://openalex.org/W1904800493, https://openalex.org/W4205239344, https://openalex.org/W2751665989 |
| referenced_works_count | 42 |
| abstract_inverted_index.a | 175, 189, 240 |
| abstract_inverted_index.12 | 95, 145 |
| abstract_inverted_index.32 | 75 |
| abstract_inverted_index.By | 49 |
| abstract_inverted_index.In | 227 |
| abstract_inverted_index.It | 80 |
| abstract_inverted_index.To | 68 |
| abstract_inverted_index.as | 6, 8, 103 |
| abstract_inverted_index.at | 94, 134, 144 |
| abstract_inverted_index.by | 99, 205 |
| abstract_inverted_index.in | 66, 178 |
| abstract_inverted_index.is | 58, 239 |
| abstract_inverted_index.it | 31 |
| abstract_inverted_index.of | 18, 54, 72, 122, 125, 199, 210, 250 |
| abstract_inverted_index.on | 171 |
| abstract_inverted_index.to | 4, 33, 109, 127, 187, 243 |
| abstract_inverted_index.478 | 135 |
| abstract_inverted_index.ETB | 39, 92, 119, 142, 194 |
| abstract_inverted_index.The | 0, 24, 38, 113, 166, 180, 197 |
| abstract_inverted_index.and | 22, 27, 106, 141, 160, 248 |
| abstract_inverted_index.are | 64 |
| abstract_inverted_index.has | 184, 202 |
| abstract_inverted_index.how | 83 |
| abstract_inverted_index.its | 222 |
| abstract_inverted_index.the | 35, 55, 70, 73, 84, 107, 193, 200, 206, 218, 229, 245 |
| abstract_inverted_index.was | 16, 42, 78, 81 |
| abstract_inverted_index.(EC) | 21, 130 |
| abstract_inverted_index.(F0) | 120 |
| abstract_inverted_index.Cmax | 191 |
| abstract_inverted_index.ETB. | 251 |
| abstract_inverted_index.This | 214 |
| abstract_inverted_index.been | 185, 203 |
| abstract_inverted_index.drug | 234 |
| abstract_inverted_index.even | 224 |
| abstract_inverted_index.from | 147 |
| abstract_inverted_index.have | 188 |
| abstract_inverted_index.like | 87 |
| abstract_inverted_index.make | 30 |
| abstract_inverted_index.rate | 133 |
| abstract_inverted_index.such | 102 |
| abstract_inverted_index.than | 192 |
| abstract_inverted_index.that | 15, 63, 217, 233 |
| abstract_inverted_index.this | 50 |
| abstract_inverted_index.well | 7 |
| abstract_inverted_index.were | 97, 150 |
| abstract_inverted_index.with | 60, 118, 131 |
| abstract_inverted_index.% | 164 |
| abstract_inverted_index.h | 96, 146 |
| abstract_inverted_index.(ETB) | 14 |
| abstract_inverted_index.1:2.8 | 123 |
| abstract_inverted_index.82.36 | 156 |
| abstract_inverted_index.85.49 | 161 |
| abstract_inverted_index.after | 225 |
| abstract_inverted_index.aimed | 3 |
| abstract_inverted_index.based | 237 |
| abstract_inverted_index.ethyl | 19, 110, 128 |
| abstract_inverted_index.found | 186 |
| abstract_inverted_index.model | 173 |
| abstract_inverted_index.ratio | 124 |
| abstract_inverted_index.shown | 151 |
| abstract_inverted_index.shows | 174 |
| abstract_inverted_index.speed | 105 |
| abstract_inverted_index.study | 169, 209, 215 |
| abstract_inverted_index.using | 44 |
| abstract_inverted_index.water | 25 |
| abstract_inverted_index.%, | 159 |
| abstract_inverted_index.± | 153, 157, 162 |
| abstract_inverted_index.104.89 | 152 |
| abstract_inverted_index.agents | 62 |
| abstract_inverted_index.design | 69, 77 |
| abstract_inverted_index.easier | 32 |
| abstract_inverted_index.higher | 190 |
| abstract_inverted_index.loaded | 40, 117, 235 |
| abstract_inverted_index.method | 242 |
| abstract_inverted_index.pectin | 108, 126 |
| abstract_inverted_index.rabbit | 172 |
| abstract_inverted_index.ratio. | 112 |
| abstract_inverted_index.water. | 67 |
| abstract_inverted_index.nm, | 155 |
| abstract_inverted_index.90days. | 226 |
| abstract_inverted_index.In-vivo | 167 |
| abstract_inverted_index.aqueous | 195 |
| abstract_inverted_index.develop | 5 |
| abstract_inverted_index.improve | 244 |
| abstract_inverted_index.optimal | 114 |
| abstract_inverted_index.organic | 52 |
| abstract_inverted_index.pectin. | 23 |
| abstract_inverted_index.present | 1, 230 |
| abstract_inverted_index.primary | 56 |
| abstract_inverted_index.release | 143 |
| abstract_inverted_index.soluble | 65 |
| abstract_inverted_index.solvent | 46 |
| abstract_inverted_index.0.62 | 154 |
| abstract_inverted_index.1.84 | 163 |
| abstract_inverted_index.2.85 | 158 |
| abstract_inverted_index.rpm. | 136 |
| abstract_inverted_index.Particle | 137 |
| abstract_inverted_index.composed | 17, 121 |
| abstract_inverted_index.efficacy | 247 |
| abstract_inverted_index.general, | 228 |
| abstract_inverted_index.optimize | 9 |
| abstract_inverted_index.particle | 88 |
| abstract_inverted_index.process. | 48 |
| abstract_inverted_index.response | 85 |
| abstract_inverted_index.retained | 221 |
| abstract_inverted_index.rotation | 104 |
| abstract_inverted_index.solution | 53 |
| abstract_inverted_index.stirring | 132 |
| abstract_inverted_index.suitable | 241 |
| abstract_inverted_index.cellulose | 20, 111, 129 |
| abstract_inverted_index.component | 57 |
| abstract_inverted_index.conducted | 170 |
| abstract_inverted_index.diffusion | 47, 93 |
| abstract_inverted_index.enzymatic | 28 |
| abstract_inverted_index.erlotinib | 12 |
| abstract_inverted_index.fabricate | 34 |
| abstract_inverted_index.factorial | 76 |
| abstract_inverted_index.formation | 71 |
| abstract_inverted_index.indicated | 216 |
| abstract_inverted_index.optimized | 148, 181, 211, 219 |
| abstract_inverted_index.stability | 198, 208, 223 |
| abstract_inverted_index.variables | 86, 101 |
| abstract_inverted_index.containing | 11 |
| abstract_inverted_index.determined | 204 |
| abstract_inverted_index.dimension, | 89, 138 |
| abstract_inverted_index.emulsified | 59 |
| abstract_inverted_index.entrapment | 90, 139 |
| abstract_inverted_index.influenced | 98 |
| abstract_inverted_index.solubility | 26 |
| abstract_inverted_index.technique, | 51 |
| abstract_inverted_index.accelerated | 207 |
| abstract_inverted_index.efficiency, | 91, 140 |
| abstract_inverted_index.formulation | 116, 149, 183, 201, 220, 238 |
| abstract_inverted_index.improvement | 177 |
| abstract_inverted_index.independent | 100 |
| abstract_inverted_index.microsponge | 10, 36, 41, 115, 182, 212, 236 |
| abstract_inverted_index.significant | 176 |
| abstract_inverted_index.stabilizing | 61 |
| abstract_inverted_index.suspension. | 196 |
| abstract_inverted_index.therapeutic | 246 |
| abstract_inverted_index.demonstrated | 232 |
| abstract_inverted_index.formulation. | 37, 213 |
| abstract_inverted_index.implemented. | 79 |
| abstract_inverted_index.investigated | 82 |
| abstract_inverted_index.manufactured | 43 |
| abstract_inverted_index.microsponge, | 74 |
| abstract_inverted_index.hydrochloride | 13 |
| abstract_inverted_index.investigation | 2, 231 |
| abstract_inverted_index.respectively. | 165 |
| abstract_inverted_index.quasi-emulsion | 45 |
| abstract_inverted_index.susceptibility | 29 |
| abstract_inverted_index.bioavailability | 249 |
| abstract_inverted_index.pharmacokinetic | 168 |
| abstract_inverted_index.bioavailability. | 179 |
| cited_by_percentile_year.max | 97 |
| cited_by_percentile_year.min | 90 |
| corresponding_author_ids | https://openalex.org/A5078051329 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 7 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/6 |
| sustainable_development_goals[0].score | 0.8100000023841858 |
| sustainable_development_goals[0].display_name | Clean water and sanitation |
| citation_normalized_percentile.value | 0.85102558 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | True |