DOP71 Effects of ustekinumab induction therapy on endoscopic and histological healing in the UNIFI Phase 3 study in ulcerative colitis Article Swipe
 YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1093/ecco-jcc/jjy222.105
YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1093/ecco-jcc/jjy222.105
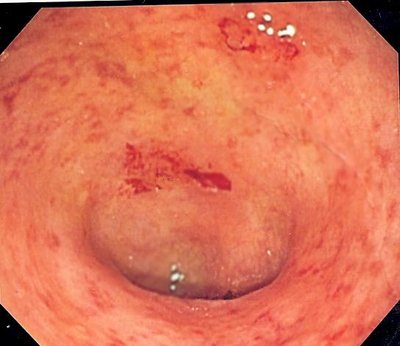
Ustekinumab (UST) is an effective therapy for moderate–severe UC; however, data regarding histological healing and the combination of histological and endoscopic healing (also described as endoscopic improvement in the appearance of the mucosa) are unknown. We evaluated the effects of UST on histological and endoscopic activity in the UNIFI Ph3 induction study of UST in moderate–severe UC(n = 961). Two colonic biopsies were collected from distal colon at screening and induction Wk8. Subjects not in response to placebo (PBO) at Wk8 received UST 6 mg/kg IV, and those not in response to UST IV received UST 90 mg SC; biopsies were obtained at Wk16. Endoscopic healing (EH; also described a endoscopic improvement in the appearance of mucosa) was defined as a Mayo endoscopy score <1; histological healing (HH) comprised the following Geboes score-based criteria: absence of erosion or ulceration, absence of crypt destruction, and <5% of crypts with epithelial neutrophil infiltration. To encompass both macro- and microscopic scales, histo-endoscopic mucosal healing (HEMH) was defined as achieving both EH and HH. At Wk8, EH was achieved in 26.6% and 13.8% of subjects treated with UST (combined 130 mg and 6 mg/kg IV doses)and PBO, respectively (adjusted tx difference, 12.8%; 95% CI, 7.9–17.8; p < 0.001). HH was achieved in 36.8% and 21.9% of UST and PBO-treated subjects, respectively (adjusted tx difference, 15.0%; 95% CI, 9.0–21.0; p < 0.001). Histo-endoscopic mucosal (HEMH) was achieved in 19.3% and 8.9% of UST and PBO-treated subjects, respectively (adjusted tx difference, 12.5%; 95% CI, 6.2–14.8; p < 0.001). Similar rates of EH, HH, and HEMH were achieved following induction with UST 130 mg or 6 mg/kg IV. Subjects not in response to PBO or UST at Wk8 were treated with UST at that time and re-evaluated at Wk16; of these, 12.1% and 16.5% of subjects who initially received UST or PBO IV, respectively, achieved HEMH. HH at Wk8 or Wk16 (irrespective of induction tx) was significantly associated with EH and HEMH (p < 0.001) and with both absolute levels and post-tx changes in Mayo score, partial Mayo score, and Mayo symptom sub-scores for stool frequency and rectal bleeding. Table 1. Clinical outcomes for subjects with or without histological healing at Week 8 and Week 16 in the UNIFI Phase 3 induction study Among subjects with moderately–severely active UC, those receiving IV UST induction had higher rates of EH, HH, and HEMH than those receiving PBO. Approximately 10% of subjects who did not achieve clinical response 8 weeks after IV UST achieved HEMH following a second (SC) dose. HH is associated with reductions in clinical and endoscopic disease activity as well as patient-reported symptoms.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1093/ecco-jcc/jjy222.105
- https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdf
- OA Status
- bronze
- Cited By
- 5
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W2913845025
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W2913845025Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1093/ecco-jcc/jjy222.105Digital Object Identifier
- Title
-
DOP71 Effects of ustekinumab induction therapy on endoscopic and histological healing in the UNIFI Phase 3 study in ulcerative colitisWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2019Year of publication
- Publication date
-
2019-01-25Full publication date if available
- Authors
-
K Li, Joshua R. Friedman, Colleen Marano, H Zhang, Feifei Yang, Brian G. Feagan, Laurent Peyrin‐Biroulet, Gert De HertoghList of authors in order
- Landing page
-
https://doi.org/10.1093/ecco-jcc/jjy222.105Publisher landing page
- PDF URL
-
https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
bronzeOpen access status per OpenAlex
- OA URL
-
https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdfDirect OA link when available
- Concepts
-
Ustekinumab, Ulcerative colitis, Medicine, Colitis, Internal medicine, Gastroenterology, Dermatology, Disease, InfliximabTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
5Total citation count in OpenAlex
- Citations by year (recent)
-
2024: 1, 2019: 4Per-year citation counts (last 5 years)
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W2913845025 |
|---|---|
| doi | https://doi.org/10.1093/ecco-jcc/jjy222.105 |
| ids.doi | https://doi.org/10.1093/ecco-jcc/jjy222.105 |
| ids.mag | 2913845025 |
| ids.openalex | https://openalex.org/W2913845025 |
| fwci | 0.71050101 |
| type | article |
| title | DOP71 Effects of ustekinumab induction therapy on endoscopic and histological healing in the UNIFI Phase 3 study in ulcerative colitis |
| biblio.issue | Supplement_1 |
| biblio.volume | 13 |
| biblio.last_page | S073 |
| biblio.first_page | S073 |
| topics[0].id | https://openalex.org/T10134 |
| topics[0].field.id | https://openalex.org/fields/13 |
| topics[0].field.display_name | Biochemistry, Genetics and Molecular Biology |
| topics[0].score | 0.9988999962806702 |
| topics[0].domain.id | https://openalex.org/domains/1 |
| topics[0].domain.display_name | Life Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/1311 |
| topics[0].subfield.display_name | Genetics |
| topics[0].display_name | Inflammatory Bowel Disease |
| topics[1].id | https://openalex.org/T13569 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9940999746322632 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2713 |
| topics[1].subfield.display_name | Epidemiology |
| topics[1].display_name | Microscopic Colitis |
| topics[2].id | https://openalex.org/T12064 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9595000147819519 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2746 |
| topics[2].subfield.display_name | Surgery |
| topics[2].display_name | Intraperitoneal and Appendiceal Malignancies |
| is_xpac | False |
| apc_list.value | 3881 |
| apc_list.currency | EUR |
| apc_list.value_usd | 4185 |
| apc_paid | |
| concepts[0].id | https://openalex.org/C2778975655 |
| concepts[0].level | 4 |
| concepts[0].score | 0.9035452604293823 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q420305 |
| concepts[0].display_name | Ustekinumab |
| concepts[1].id | https://openalex.org/C2780479503 |
| concepts[1].level | 3 |
| concepts[1].score | 0.8455452919006348 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q1477 |
| concepts[1].display_name | Ulcerative colitis |
| concepts[2].id | https://openalex.org/C71924100 |
| concepts[2].level | 0 |
| concepts[2].score | 0.6539590954780579 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[2].display_name | Medicine |
| concepts[3].id | https://openalex.org/C2775862500 |
| concepts[3].level | 2 |
| concepts[3].score | 0.4634297788143158 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q2453464 |
| concepts[3].display_name | Colitis |
| concepts[4].id | https://openalex.org/C126322002 |
| concepts[4].level | 1 |
| concepts[4].score | 0.40945136547088623 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[4].display_name | Internal medicine |
| concepts[5].id | https://openalex.org/C90924648 |
| concepts[5].level | 1 |
| concepts[5].score | 0.3833279013633728 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q120569 |
| concepts[5].display_name | Gastroenterology |
| concepts[6].id | https://openalex.org/C16005928 |
| concepts[6].level | 1 |
| concepts[6].score | 0.34436166286468506 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q171171 |
| concepts[6].display_name | Dermatology |
| concepts[7].id | https://openalex.org/C2779134260 |
| concepts[7].level | 2 |
| concepts[7].score | 0.19113695621490479 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q12136 |
| concepts[7].display_name | Disease |
| concepts[8].id | https://openalex.org/C2777138892 |
| concepts[8].level | 3 |
| concepts[8].score | 0.0 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q415264 |
| concepts[8].display_name | Infliximab |
| keywords[0].id | https://openalex.org/keywords/ustekinumab |
| keywords[0].score | 0.9035452604293823 |
| keywords[0].display_name | Ustekinumab |
| keywords[1].id | https://openalex.org/keywords/ulcerative-colitis |
| keywords[1].score | 0.8455452919006348 |
| keywords[1].display_name | Ulcerative colitis |
| keywords[2].id | https://openalex.org/keywords/medicine |
| keywords[2].score | 0.6539590954780579 |
| keywords[2].display_name | Medicine |
| keywords[3].id | https://openalex.org/keywords/colitis |
| keywords[3].score | 0.4634297788143158 |
| keywords[3].display_name | Colitis |
| keywords[4].id | https://openalex.org/keywords/internal-medicine |
| keywords[4].score | 0.40945136547088623 |
| keywords[4].display_name | Internal medicine |
| keywords[5].id | https://openalex.org/keywords/gastroenterology |
| keywords[5].score | 0.3833279013633728 |
| keywords[5].display_name | Gastroenterology |
| keywords[6].id | https://openalex.org/keywords/dermatology |
| keywords[6].score | 0.34436166286468506 |
| keywords[6].display_name | Dermatology |
| keywords[7].id | https://openalex.org/keywords/disease |
| keywords[7].score | 0.19113695621490479 |
| keywords[7].display_name | Disease |
| language | en |
| locations[0].id | doi:10.1093/ecco-jcc/jjy222.105 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S2735960814 |
| locations[0].source.issn | 1873-9946, 1876-4479 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1873-9946 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Journal of Crohn s and Colitis |
| locations[0].source.host_organization | https://openalex.org/P4310311648 |
| locations[0].source.host_organization_name | Oxford University Press |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310311648 |
| locations[0].license | |
| locations[0].pdf_url | https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Journal of Crohn's and Colitis |
| locations[0].landing_page_url | https://doi.org/10.1093/ecco-jcc/jjy222.105 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A5018278447 |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | K Li |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I4210151386 |
| authorships[0].affiliations[0].raw_affiliation_string | Janssen Research and Development, LLC, Spring House, USA |
| authorships[0].institutions[0].id | https://openalex.org/I4210151386 |
| authorships[0].institutions[0].ror | https://ror.org/05af73403 |
| authorships[0].institutions[0].type | company |
| authorships[0].institutions[0].lineage | https://openalex.org/I1330063522, https://openalex.org/I4210151386 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | Janssen (United States) |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | K Li |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Janssen Research and Development, LLC, Spring House, USA |
| authorships[1].author.id | https://openalex.org/A5011427142 |
| authorships[1].author.orcid | https://orcid.org/0000-0001-9382-8429 |
| authorships[1].author.display_name | Joshua R. Friedman |
| authorships[1].countries | US |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I4210151386 |
| authorships[1].affiliations[0].raw_affiliation_string | Janssen Research and Development, LLC, Spring House, USA |
| authorships[1].institutions[0].id | https://openalex.org/I4210151386 |
| authorships[1].institutions[0].ror | https://ror.org/05af73403 |
| authorships[1].institutions[0].type | company |
| authorships[1].institutions[0].lineage | https://openalex.org/I1330063522, https://openalex.org/I4210151386 |
| authorships[1].institutions[0].country_code | US |
| authorships[1].institutions[0].display_name | Janssen (United States) |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | J R Friedman |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Janssen Research and Development, LLC, Spring House, USA |
| authorships[2].author.id | https://openalex.org/A5052046238 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-4965-5142 |
| authorships[2].author.display_name | Colleen Marano |
| authorships[2].countries | US |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I4210151386 |
| authorships[2].affiliations[0].raw_affiliation_string | Janssen Research and Development, LLC, Spring House, USA |
| authorships[2].institutions[0].id | https://openalex.org/I4210151386 |
| authorships[2].institutions[0].ror | https://ror.org/05af73403 |
| authorships[2].institutions[0].type | company |
| authorships[2].institutions[0].lineage | https://openalex.org/I1330063522, https://openalex.org/I4210151386 |
| authorships[2].institutions[0].country_code | US |
| authorships[2].institutions[0].display_name | Janssen (United States) |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | C Marano |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Janssen Research and Development, LLC, Spring House, USA |
| authorships[3].author.id | https://openalex.org/A5035601572 |
| authorships[3].author.orcid | https://orcid.org/0009-0007-7202-9397 |
| authorships[3].author.display_name | H Zhang |
| authorships[3].countries | US |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I4210151386 |
| authorships[3].affiliations[0].raw_affiliation_string | Janssen Research and Development, LLC, Spring House, USA |
| authorships[3].institutions[0].id | https://openalex.org/I4210151386 |
| authorships[3].institutions[0].ror | https://ror.org/05af73403 |
| authorships[3].institutions[0].type | company |
| authorships[3].institutions[0].lineage | https://openalex.org/I1330063522, https://openalex.org/I4210151386 |
| authorships[3].institutions[0].country_code | US |
| authorships[3].institutions[0].display_name | Janssen (United States) |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | H Zhang |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Janssen Research and Development, LLC, Spring House, USA |
| authorships[4].author.id | https://openalex.org/A5031302163 |
| authorships[4].author.orcid | https://orcid.org/0000-0001-9362-7635 |
| authorships[4].author.display_name | Feifei Yang |
| authorships[4].countries | US |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I4210151386 |
| authorships[4].affiliations[0].raw_affiliation_string | Janssen Research and Development, LLC, Spring House, USA |
| authorships[4].institutions[0].id | https://openalex.org/I4210151386 |
| authorships[4].institutions[0].ror | https://ror.org/05af73403 |
| authorships[4].institutions[0].type | company |
| authorships[4].institutions[0].lineage | https://openalex.org/I1330063522, https://openalex.org/I4210151386 |
| authorships[4].institutions[0].country_code | US |
| authorships[4].institutions[0].display_name | Janssen (United States) |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | F Yang |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Janssen Research and Development, LLC, Spring House, USA |
| authorships[5].author.id | https://openalex.org/A5048464948 |
| authorships[5].author.orcid | https://orcid.org/0000-0002-6914-3822 |
| authorships[5].author.display_name | Brian G. Feagan |
| authorships[5].countries | CA |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I4210105758 |
| authorships[5].affiliations[0].raw_affiliation_string | Robarts Research Institute, Robarts Clinical Trials, London, Canada |
| authorships[5].institutions[0].id | https://openalex.org/I4210105758 |
| authorships[5].institutions[0].ror | https://ror.org/01e36dv41 |
| authorships[5].institutions[0].type | facility |
| authorships[5].institutions[0].lineage | https://openalex.org/I125749732, https://openalex.org/I4210105758, https://openalex.org/I4405252475 |
| authorships[5].institutions[0].country_code | CA |
| authorships[5].institutions[0].display_name | Robarts Clinical Trials |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | B G Feagan |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Robarts Research Institute, Robarts Clinical Trials, London, Canada |
| authorships[6].author.id | https://openalex.org/A5042884495 |
| authorships[6].author.orcid | https://orcid.org/0000-0003-2536-6618 |
| authorships[6].author.display_name | Laurent Peyrin‐Biroulet |
| authorships[6].countries | FR |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I90183372 |
| authorships[6].affiliations[0].raw_affiliation_string | Nancy University Hospital, Université de Lorraine, Nancy, France |
| authorships[6].institutions[0].id | https://openalex.org/I90183372 |
| authorships[6].institutions[0].ror | https://ror.org/04vfs2w97 |
| authorships[6].institutions[0].type | education |
| authorships[6].institutions[0].lineage | https://openalex.org/I90183372 |
| authorships[6].institutions[0].country_code | FR |
| authorships[6].institutions[0].display_name | Université de Lorraine |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | L Peyrin-Biroulet |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Nancy University Hospital, Université de Lorraine, Nancy, France |
| authorships[7].author.id | https://openalex.org/A5003632939 |
| authorships[7].author.orcid | https://orcid.org/0000-0001-8494-7725 |
| authorships[7].author.display_name | Gert De Hertogh |
| authorships[7].countries | BE |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I99464096 |
| authorships[7].affiliations[0].raw_affiliation_string | University Hospitals KU, Leuven, Belgium |
| authorships[7].institutions[0].id | https://openalex.org/I99464096 |
| authorships[7].institutions[0].ror | https://ror.org/05f950310 |
| authorships[7].institutions[0].type | education |
| authorships[7].institutions[0].lineage | https://openalex.org/I99464096 |
| authorships[7].institutions[0].country_code | BE |
| authorships[7].institutions[0].display_name | KU Leuven |
| authorships[7].author_position | last |
| authorships[7].raw_author_name | G De Hertogh |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | University Hospitals KU, Leuven, Belgium |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdf |
| open_access.oa_status | bronze |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | DOP71 Effects of ustekinumab induction therapy on endoscopic and histological healing in the UNIFI Phase 3 study in ulcerative colitis |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10134 |
| primary_topic.field.id | https://openalex.org/fields/13 |
| primary_topic.field.display_name | Biochemistry, Genetics and Molecular Biology |
| primary_topic.score | 0.9988999962806702 |
| primary_topic.domain.id | https://openalex.org/domains/1 |
| primary_topic.domain.display_name | Life Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/1311 |
| primary_topic.subfield.display_name | Genetics |
| primary_topic.display_name | Inflammatory Bowel Disease |
| related_works | https://openalex.org/W2582131160, https://openalex.org/W2488600916, https://openalex.org/W1995555245, https://openalex.org/W3194385729, https://openalex.org/W2999643437, https://openalex.org/W2790483177, https://openalex.org/W4255303081, https://openalex.org/W2802439938, https://openalex.org/W2048986416, https://openalex.org/W71416396 |
| cited_by_count | 5 |
| counts_by_year[0].year | 2024 |
| counts_by_year[0].cited_by_count | 1 |
| counts_by_year[1].year | 2019 |
| counts_by_year[1].cited_by_count | 4 |
| locations_count | 1 |
| best_oa_location.id | doi:10.1093/ecco-jcc/jjy222.105 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S2735960814 |
| best_oa_location.source.issn | 1873-9946, 1876-4479 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | 1873-9946 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | Journal of Crohn s and Colitis |
| best_oa_location.source.host_organization | https://openalex.org/P4310311648 |
| best_oa_location.source.host_organization_name | Oxford University Press |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310311648 |
| best_oa_location.license | |
| best_oa_location.pdf_url | https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Journal of Crohn's and Colitis |
| best_oa_location.landing_page_url | https://doi.org/10.1093/ecco-jcc/jjy222.105 |
| primary_location.id | doi:10.1093/ecco-jcc/jjy222.105 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S2735960814 |
| primary_location.source.issn | 1873-9946, 1876-4479 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1873-9946 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Journal of Crohn s and Colitis |
| primary_location.source.host_organization | https://openalex.org/P4310311648 |
| primary_location.source.host_organization_name | Oxford University Press |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310311648 |
| primary_location.license | |
| primary_location.pdf_url | https://academic.oup.com/ecco-jcc/article-pdf/13/Supplement_1/S073/27599190/jjy222.105.pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Journal of Crohn's and Colitis |
| primary_location.landing_page_url | https://doi.org/10.1093/ecco-jcc/jjy222.105 |
| publication_date | 2019-01-25 |
| publication_year | 2019 |
| referenced_works_count | 0 |
| abstract_inverted_index.3 | 373 |
| abstract_inverted_index.6 | 83, 188, 268 |
| abstract_inverted_index.8 | 365, 409 |
| abstract_inverted_index.< | 202, 225, 250, 326 |
| abstract_inverted_index.= | 57 |
| abstract_inverted_index.a | 109, 120, 417 |
| abstract_inverted_index.p | 201, 224, 249 |
| abstract_inverted_index.(p | 325 |
| abstract_inverted_index.1. | 353 |
| abstract_inverted_index.16 | 368 |
| abstract_inverted_index.90 | 96 |
| abstract_inverted_index.At | 170 |
| abstract_inverted_index.EH | 167, 172, 322 |
| abstract_inverted_index.HH | 204, 309, 421 |
| abstract_inverted_index.IV | 93, 190, 384, 412 |
| abstract_inverted_index.To | 151 |
| abstract_inverted_index.We | 35 |
| abstract_inverted_index.an | 3 |
| abstract_inverted_index.as | 24, 119, 164, 432, 434 |
| abstract_inverted_index.at | 67, 79, 102, 279, 285, 290, 310, 363 |
| abstract_inverted_index.in | 27, 46, 54, 74, 89, 112, 175, 207, 232, 273, 336, 369, 426 |
| abstract_inverted_index.is | 2, 422 |
| abstract_inverted_index.mg | 97, 186, 266 |
| abstract_inverted_index.of | 17, 30, 39, 52, 115, 135, 140, 145, 179, 211, 236, 254, 292, 297, 315, 390, 401 |
| abstract_inverted_index.on | 41 |
| abstract_inverted_index.or | 137, 267, 277, 303, 312, 359 |
| abstract_inverted_index.to | 76, 91, 275 |
| abstract_inverted_index.tx | 195, 218, 243 |
| abstract_inverted_index.10% | 400 |
| abstract_inverted_index.130 | 185, 265 |
| abstract_inverted_index.95% | 198, 221, 246 |
| abstract_inverted_index.<1; | 124 |
| abstract_inverted_index.<5% | 144 |
| abstract_inverted_index.CI, | 199, 222, 247 |
| abstract_inverted_index.EH, | 255, 391 |
| abstract_inverted_index.HH, | 256, 392 |
| abstract_inverted_index.HH. | 169 |
| abstract_inverted_index.IV, | 85, 305 |
| abstract_inverted_index.IV. | 270 |
| abstract_inverted_index.PBO | 276, 304 |
| abstract_inverted_index.Ph3 | 49 |
| abstract_inverted_index.SC; | 98 |
| abstract_inverted_index.Two | 59 |
| abstract_inverted_index.UC, | 381 |
| abstract_inverted_index.UC; | 8 |
| abstract_inverted_index.UST | 40, 53, 82, 92, 95, 183, 212, 237, 264, 278, 284, 302, 385, 413 |
| abstract_inverted_index.Wk8 | 80, 280, 311 |
| abstract_inverted_index.and | 14, 19, 43, 69, 86, 143, 155, 168, 177, 187, 209, 213, 234, 238, 257, 288, 295, 323, 328, 333, 342, 349, 366, 393, 428 |
| abstract_inverted_index.are | 33 |
| abstract_inverted_index.did | 404 |
| abstract_inverted_index.for | 6, 346, 356 |
| abstract_inverted_index.had | 387 |
| abstract_inverted_index.not | 73, 88, 272, 405 |
| abstract_inverted_index.the | 15, 28, 31, 37, 47, 113, 129, 370 |
| abstract_inverted_index.tx) | 317 |
| abstract_inverted_index.was | 117, 162, 173, 205, 230, 318 |
| abstract_inverted_index.who | 299, 403 |
| abstract_inverted_index.(EH; | 106 |
| abstract_inverted_index.(HH) | 127 |
| abstract_inverted_index.(SC) | 419 |
| abstract_inverted_index.8.9% | 235 |
| abstract_inverted_index.HEMH | 258, 324, 394, 415 |
| abstract_inverted_index.Mayo | 121, 337, 340, 343 |
| abstract_inverted_index.PBO, | 192 |
| abstract_inverted_index.PBO. | 398 |
| abstract_inverted_index.UC(n | 56 |
| abstract_inverted_index.Week | 364, 367 |
| abstract_inverted_index.Wk16 | 313 |
| abstract_inverted_index.Wk8, | 171 |
| abstract_inverted_index.Wk8. | 71 |
| abstract_inverted_index.also | 107 |
| abstract_inverted_index.both | 153, 166, 330 |
| abstract_inverted_index.data | 10 |
| abstract_inverted_index.from | 64 |
| abstract_inverted_index.than | 395 |
| abstract_inverted_index.that | 286 |
| abstract_inverted_index.time | 287 |
| abstract_inverted_index.well | 433 |
| abstract_inverted_index.were | 62, 100, 259, 281 |
| abstract_inverted_index.with | 147, 182, 263, 283, 321, 329, 358, 378, 424 |
| abstract_inverted_index.(PBO) | 78 |
| abstract_inverted_index.(UST) | 1 |
| abstract_inverted_index.(also | 22 |
| abstract_inverted_index.12.1% | 294 |
| abstract_inverted_index.13.8% | 178 |
| abstract_inverted_index.16.5% | 296 |
| abstract_inverted_index.19.3% | 233 |
| abstract_inverted_index.21.9% | 210 |
| abstract_inverted_index.26.6% | 176 |
| abstract_inverted_index.36.8% | 208 |
| abstract_inverted_index.961). | 58 |
| abstract_inverted_index.Among | 376 |
| abstract_inverted_index.HEMH. | 308 |
| abstract_inverted_index.Phase | 372 |
| abstract_inverted_index.Table | 352 |
| abstract_inverted_index.UNIFI | 48, 371 |
| abstract_inverted_index.Wk16. | 103 |
| abstract_inverted_index.Wk16; | 291 |
| abstract_inverted_index.after | 411 |
| abstract_inverted_index.colon | 66 |
| abstract_inverted_index.crypt | 141 |
| abstract_inverted_index.dose. | 420 |
| abstract_inverted_index.mg/kg | 84, 189, 269 |
| abstract_inverted_index.rates | 253, 389 |
| abstract_inverted_index.score | 123 |
| abstract_inverted_index.stool | 347 |
| abstract_inverted_index.study | 51, 375 |
| abstract_inverted_index.those | 87, 382, 396 |
| abstract_inverted_index.weeks | 410 |
| abstract_inverted_index.(HEMH) | 161, 229 |
| abstract_inverted_index.0.001) | 327 |
| abstract_inverted_index.12.5%; | 245 |
| abstract_inverted_index.12.8%; | 197 |
| abstract_inverted_index.15.0%; | 220 |
| abstract_inverted_index.Geboes | 131 |
| abstract_inverted_index.active | 380 |
| abstract_inverted_index.crypts | 146 |
| abstract_inverted_index.distal | 65 |
| abstract_inverted_index.higher | 388 |
| abstract_inverted_index.levels | 332 |
| abstract_inverted_index.macro- | 154 |
| abstract_inverted_index.rectal | 350 |
| abstract_inverted_index.score, | 338, 341 |
| abstract_inverted_index.second | 418 |
| abstract_inverted_index.these, | 293 |
| abstract_inverted_index.0.001). | 203, 226, 251 |
| abstract_inverted_index.Similar | 252 |
| abstract_inverted_index.absence | 134, 139 |
| abstract_inverted_index.achieve | 406 |
| abstract_inverted_index.changes | 335 |
| abstract_inverted_index.colonic | 60 |
| abstract_inverted_index.defined | 118, 163 |
| abstract_inverted_index.disease | 430 |
| abstract_inverted_index.effects | 38 |
| abstract_inverted_index.erosion | 136 |
| abstract_inverted_index.healing | 13, 21, 105, 126, 160, 362 |
| abstract_inverted_index.mucosa) | 32, 116 |
| abstract_inverted_index.mucosal | 159, 228 |
| abstract_inverted_index.partial | 339 |
| abstract_inverted_index.placebo | 77 |
| abstract_inverted_index.post-tx | 334 |
| abstract_inverted_index.scales, | 157 |
| abstract_inverted_index.symptom | 344 |
| abstract_inverted_index.therapy | 5 |
| abstract_inverted_index.treated | 181, 282 |
| abstract_inverted_index.without | 360 |
| abstract_inverted_index.Clinical | 354 |
| abstract_inverted_index.Subjects | 72, 271 |
| abstract_inverted_index.absolute | 331 |
| abstract_inverted_index.achieved | 174, 206, 231, 260, 307, 414 |
| abstract_inverted_index.activity | 45, 431 |
| abstract_inverted_index.biopsies | 61, 99 |
| abstract_inverted_index.clinical | 407, 427 |
| abstract_inverted_index.however, | 9 |
| abstract_inverted_index.obtained | 101 |
| abstract_inverted_index.outcomes | 355 |
| abstract_inverted_index.received | 81, 94, 301 |
| abstract_inverted_index.response | 75, 90, 274, 408 |
| abstract_inverted_index.subjects | 180, 298, 357, 377, 402 |
| abstract_inverted_index.unknown. | 34 |
| abstract_inverted_index.(adjusted | 194, 217, 242 |
| abstract_inverted_index.(combined | 184 |
| abstract_inverted_index.achieving | 165 |
| abstract_inverted_index.bleeding. | 351 |
| abstract_inverted_index.collected | 63 |
| abstract_inverted_index.comprised | 128 |
| abstract_inverted_index.criteria: | 133 |
| abstract_inverted_index.described | 23, 108 |
| abstract_inverted_index.doses)and | 191 |
| abstract_inverted_index.effective | 4 |
| abstract_inverted_index.encompass | 152 |
| abstract_inverted_index.endoscopy | 122 |
| abstract_inverted_index.evaluated | 36 |
| abstract_inverted_index.following | 130, 261, 416 |
| abstract_inverted_index.frequency | 348 |
| abstract_inverted_index.induction | 50, 70, 262, 316, 374, 386 |
| abstract_inverted_index.initially | 300 |
| abstract_inverted_index.receiving | 383, 397 |
| abstract_inverted_index.regarding | 11 |
| abstract_inverted_index.screening | 68 |
| abstract_inverted_index.subjects, | 215, 240 |
| abstract_inverted_index.symptoms. | 436 |
| abstract_inverted_index.Endoscopic | 104 |
| abstract_inverted_index.appearance | 29, 114 |
| abstract_inverted_index.associated | 320, 423 |
| abstract_inverted_index.endoscopic | 20, 25, 44, 110, 429 |
| abstract_inverted_index.epithelial | 148 |
| abstract_inverted_index.neutrophil | 149 |
| abstract_inverted_index.reductions | 425 |
| abstract_inverted_index.sub-scores | 345 |
| abstract_inverted_index.6.2–14.8; | 248 |
| abstract_inverted_index.7.9–17.8; | 200 |
| abstract_inverted_index.9.0–21.0; | 223 |
| abstract_inverted_index.PBO-treated | 214, 239 |
| abstract_inverted_index.Ustekinumab | 0 |
| abstract_inverted_index.combination | 16 |
| abstract_inverted_index.difference, | 196, 219, 244 |
| abstract_inverted_index.improvement | 26, 111 |
| abstract_inverted_index.microscopic | 156 |
| abstract_inverted_index.score-based | 132 |
| abstract_inverted_index.ulceration, | 138 |
| abstract_inverted_index.destruction, | 142 |
| abstract_inverted_index.histological | 12, 18, 42, 125, 361 |
| abstract_inverted_index.re-evaluated | 289 |
| abstract_inverted_index.respectively | 193, 216, 241 |
| abstract_inverted_index.(irrespective | 314 |
| abstract_inverted_index.Approximately | 399 |
| abstract_inverted_index.infiltration. | 150 |
| abstract_inverted_index.respectively, | 306 |
| abstract_inverted_index.significantly | 319 |
| abstract_inverted_index.Histo-endoscopic | 227 |
| abstract_inverted_index.histo-endoscopic | 158 |
| abstract_inverted_index.patient-reported | 435 |
| abstract_inverted_index.moderate–severe | 7, 55 |
| abstract_inverted_index.moderately–severely | 379 |
| cited_by_percentile_year.max | 98 |
| cited_by_percentile_year.min | 90 |
| countries_distinct_count | 4 |
| institutions_distinct_count | 8 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/2 |
| sustainable_development_goals[0].score | 0.6299999952316284 |
| sustainable_development_goals[0].display_name | Zero hunger |
| citation_normalized_percentile.value | 0.74153097 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |