Early switch from intravenous to oral antibiotic therapy in patients with cancer who have low-risk neutropenic sepsis (the EASI-SWITCH trial): study protocol for a randomised controlled trial Article Swipe
 YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.1186/s13063-020-04241-1
YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.1186/s13063-020-04241-1
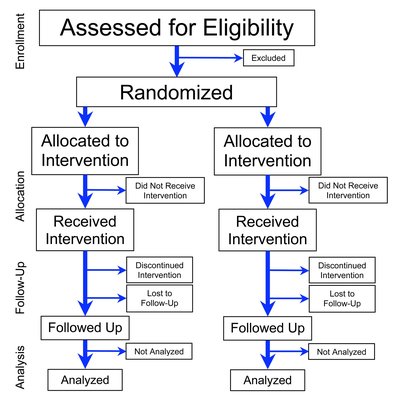
Background Neutropenic sepsis remains a common treatment complication for patients receiving systemic anti-cancer treatment. The UK National Institute for Health and Care Excellence have not recommended switching from empirical intravenous antibiotics to oral antibiotics within 48 h for patients assessed as low risk for septic complications because of uncertainty about whether this would achieve comparable outcomes to using intravenous antibiotics for longer. The UK National Institute for Health Research funded the EASI-SWITCH trial to tackle this uncertainty. Methods The trial is a pragmatic, randomised, non-inferiority trial that aims to establish the clinical and cost-effectiveness of early switching from intravenous to oral antibiotics in cancer patients with low-risk neutropenic sepsis. Patients ≥ 16 years, receiving systemic anti-cancer treatment (acute leukaemics/stem cell transplants excluded), with a temperature of > 38 °C, neutrophil count ≤ 1.0 × 10 9 /L, MASCC (Multinational Association of Supportive Care in Cancer) score ≥ 21 and receiving IV piperacillin/tazobactam or meropenem for less than 24 h are eligible to participate. Patients are randomised 1:1 either (i) to switch to oral ciprofloxacin and co-amoxiclav within 12–24 h of commencing intravenous antibiotics, completing at least 5 days total antibiotics (intervention), or (ii) to continue intravenous antibiotics for at least 48 h, with ongoing antibiotics being continued at the physician’s discretion (control). Patients are discharged home when their physician deems it appropriate. The primary outcome measure is a composite of treatment failures as assessed at day 14. The criteria for treatment failure include fever persistence or recurrence 72 h after starting intravenous antibiotics, escalation from protocolised antibiotics, hospital readmission related to infection/antibiotics, critical care support or death. Based on a 15% treatment failure rate in the control group and a 15% non-inferiority margin, the recruitment target is 230 patients. Discussion If the trial demonstrates non-inferiority of early switching to oral antibiotics, with potential benefits for patient quality of life and resource savings, this finding will have significant implications for the routine clinical management of those with low-risk neutropenic sepsis. Trial registration ISRCTN: 84288963. Registered on the 1 July 2015. 10.1186/ISRCTN84288963 . EudraCT: 2015-002830-35.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1186/s13063-020-04241-1
- https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1
- OA Status
- gold
- Cited By
- 5
- References
- 21
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W3032563905
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W3032563905Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1186/s13063-020-04241-1Digital Object Identifier
- Title
-
Early switch from intravenous to oral antibiotic therapy in patients with cancer who have low-risk neutropenic sepsis (the EASI-SWITCH trial): study protocol for a randomised controlled trialWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2020Year of publication
- Publication date
-
2020-05-27Full publication date if available
- Authors
-
Caroline Forde, Ronan McMullan, Mike Clarke, Richard H. Wilson, Ruth Plummer, Margaret Grayson, Clíona McDowell, Ashley Agus, Annmarie Doran, Daniel F. McAuley, Anne Thomas, Rosemary A. Barnes, Richard Adams, Ian Chau, Vicky M. CoyleList of authors in order
- Landing page
-
https://doi.org/10.1186/s13063-020-04241-1Publisher landing page
- PDF URL
-
https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1Direct link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1Direct OA link when available
- Concepts
-
Medicine, Sepsis, Intensive care medicine, Antibiotics, Randomized controlled trial, Cancer, Internal medicine, Biology, MicrobiologyTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
5Total citation count in OpenAlex
- Citations by year (recent)
-
2024: 2, 2023: 2, 2021: 1Per-year citation counts (last 5 years)
- References (count)
-
21Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W3032563905 |
|---|---|
| doi | https://doi.org/10.1186/s13063-020-04241-1 |
| ids.doi | https://doi.org/10.1186/s13063-020-04241-1 |
| ids.mag | 3032563905 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/32460818 |
| ids.openalex | https://openalex.org/W3032563905 |
| fwci | 0.30176336 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D061605 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Administration, Intravenous |
| mesh[1].qualifier_ui | |
| mesh[1].descriptor_ui | D000284 |
| mesh[1].is_major_topic | False |
| mesh[1].qualifier_name | |
| mesh[1].descriptor_name | Administration, Oral |
| mesh[2].qualifier_ui | |
| mesh[2].descriptor_ui | D019980 |
| mesh[2].is_major_topic | False |
| mesh[2].qualifier_name | |
| mesh[2].descriptor_name | Amoxicillin-Potassium Clavulanate Combination |
| mesh[3].qualifier_ui | Q000008 |
| mesh[3].descriptor_ui | D000900 |
| mesh[3].is_major_topic | False |
| mesh[3].qualifier_name | administration & dosage |
| mesh[3].descriptor_name | Anti-Bacterial Agents |
| mesh[4].qualifier_ui | Q000009 |
| mesh[4].descriptor_ui | D000900 |
| mesh[4].is_major_topic | False |
| mesh[4].qualifier_name | adverse effects |
| mesh[4].descriptor_name | Anti-Bacterial Agents |
| mesh[5].qualifier_ui | |
| mesh[5].descriptor_ui | D002939 |
| mesh[5].is_major_topic | False |
| mesh[5].qualifier_name | |
| mesh[5].descriptor_name | Ciprofloxacin |
| mesh[6].qualifier_ui | Q000191 |
| mesh[6].descriptor_ui | D003362 |
| mesh[6].is_major_topic | False |
| mesh[6].qualifier_name | economics |
| mesh[6].descriptor_name | Cost-Benefit Analysis |
| mesh[7].qualifier_ui | |
| mesh[7].descriptor_ui | D004334 |
| mesh[7].is_major_topic | False |
| mesh[7].qualifier_name | |
| mesh[7].descriptor_name | Drug Administration Schedule |
| mesh[8].qualifier_ui | |
| mesh[8].descriptor_ui | D000074099 |
| mesh[8].is_major_topic | False |
| mesh[8].qualifier_name | |
| mesh[8].descriptor_name | Equivalence Trials as Topic |
| mesh[9].qualifier_ui | |
| mesh[9].descriptor_ui | D006801 |
| mesh[9].is_major_topic | False |
| mesh[9].qualifier_name | |
| mesh[9].descriptor_name | Humans |
| mesh[10].qualifier_ui | |
| mesh[10].descriptor_ui | D000077731 |
| mesh[10].is_major_topic | False |
| mesh[10].qualifier_name | |
| mesh[10].descriptor_name | Meropenem |
| mesh[11].qualifier_ui | |
| mesh[11].descriptor_ui | D015337 |
| mesh[11].is_major_topic | False |
| mesh[11].qualifier_name | |
| mesh[11].descriptor_name | Multicenter Studies as Topic |
| mesh[12].qualifier_ui | Q000150 |
| mesh[12].descriptor_ui | D009369 |
| mesh[12].is_major_topic | False |
| mesh[12].qualifier_name | complications |
| mesh[12].descriptor_name | Neoplasms |
| mesh[13].qualifier_ui | Q000188 |
| mesh[13].descriptor_ui | D009503 |
| mesh[13].is_major_topic | False |
| mesh[13].qualifier_name | drug therapy |
| mesh[13].descriptor_name | Neutropenia |
| mesh[14].qualifier_ui | |
| mesh[14].descriptor_ui | D010878 |
| mesh[14].is_major_topic | False |
| mesh[14].qualifier_name | |
| mesh[14].descriptor_name | Piperacillin |
| mesh[15].qualifier_ui | |
| mesh[15].descriptor_ui | D064792 |
| mesh[15].is_major_topic | False |
| mesh[15].qualifier_name | |
| mesh[15].descriptor_name | Pragmatic Clinical Trials as Topic |
| mesh[16].qualifier_ui | |
| mesh[16].descriptor_ui | D011788 |
| mesh[16].is_major_topic | False |
| mesh[16].qualifier_name | |
| mesh[16].descriptor_name | Quality of Life |
| mesh[17].qualifier_ui | Q000188 |
| mesh[17].descriptor_ui | D018805 |
| mesh[17].is_major_topic | False |
| mesh[17].qualifier_name | drug therapy |
| mesh[17].descriptor_name | Sepsis |
| mesh[18].qualifier_ui | |
| mesh[18].descriptor_ui | D000078142 |
| mesh[18].is_major_topic | False |
| mesh[18].qualifier_name | |
| mesh[18].descriptor_name | Tazobactam |
| mesh[19].qualifier_ui | |
| mesh[19].descriptor_ui | D016896 |
| mesh[19].is_major_topic | False |
| mesh[19].qualifier_name | |
| mesh[19].descriptor_name | Treatment Outcome |
| mesh[20].qualifier_ui | |
| mesh[20].descriptor_ui | D061605 |
| mesh[20].is_major_topic | False |
| mesh[20].qualifier_name | |
| mesh[20].descriptor_name | Administration, Intravenous |
| mesh[21].qualifier_ui | |
| mesh[21].descriptor_ui | D000284 |
| mesh[21].is_major_topic | False |
| mesh[21].qualifier_name | |
| mesh[21].descriptor_name | Administration, Oral |
| mesh[22].qualifier_ui | |
| mesh[22].descriptor_ui | D019980 |
| mesh[22].is_major_topic | False |
| mesh[22].qualifier_name | |
| mesh[22].descriptor_name | Amoxicillin-Potassium Clavulanate Combination |
| mesh[23].qualifier_ui | Q000008 |
| mesh[23].descriptor_ui | D000900 |
| mesh[23].is_major_topic | False |
| mesh[23].qualifier_name | administration & dosage |
| mesh[23].descriptor_name | Anti-Bacterial Agents |
| mesh[24].qualifier_ui | Q000009 |
| mesh[24].descriptor_ui | D000900 |
| mesh[24].is_major_topic | False |
| mesh[24].qualifier_name | adverse effects |
| mesh[24].descriptor_name | Anti-Bacterial Agents |
| mesh[25].qualifier_ui | |
| mesh[25].descriptor_ui | D002939 |
| mesh[25].is_major_topic | False |
| mesh[25].qualifier_name | |
| mesh[25].descriptor_name | Ciprofloxacin |
| mesh[26].qualifier_ui | Q000191 |
| mesh[26].descriptor_ui | D003362 |
| mesh[26].is_major_topic | False |
| mesh[26].qualifier_name | economics |
| mesh[26].descriptor_name | Cost-Benefit Analysis |
| mesh[27].qualifier_ui | |
| mesh[27].descriptor_ui | D004334 |
| mesh[27].is_major_topic | False |
| mesh[27].qualifier_name | |
| mesh[27].descriptor_name | Drug Administration Schedule |
| mesh[28].qualifier_ui | |
| mesh[28].descriptor_ui | D000074099 |
| mesh[28].is_major_topic | False |
| mesh[28].qualifier_name | |
| mesh[28].descriptor_name | Equivalence Trials as Topic |
| mesh[29].qualifier_ui | |
| mesh[29].descriptor_ui | D006801 |
| mesh[29].is_major_topic | False |
| mesh[29].qualifier_name | |
| mesh[29].descriptor_name | Humans |
| mesh[30].qualifier_ui | |
| mesh[30].descriptor_ui | D000077731 |
| mesh[30].is_major_topic | False |
| mesh[30].qualifier_name | |
| mesh[30].descriptor_name | Meropenem |
| mesh[31].qualifier_ui | |
| mesh[31].descriptor_ui | D015337 |
| mesh[31].is_major_topic | False |
| mesh[31].qualifier_name | |
| mesh[31].descriptor_name | Multicenter Studies as Topic |
| mesh[32].qualifier_ui | Q000150 |
| mesh[32].descriptor_ui | D009369 |
| mesh[32].is_major_topic | False |
| mesh[32].qualifier_name | complications |
| mesh[32].descriptor_name | Neoplasms |
| mesh[33].qualifier_ui | Q000188 |
| mesh[33].descriptor_ui | D009503 |
| mesh[33].is_major_topic | False |
| mesh[33].qualifier_name | drug therapy |
| mesh[33].descriptor_name | Neutropenia |
| mesh[34].qualifier_ui | |
| mesh[34].descriptor_ui | D010878 |
| mesh[34].is_major_topic | False |
| mesh[34].qualifier_name | |
| mesh[34].descriptor_name | Piperacillin |
| mesh[35].qualifier_ui | |
| mesh[35].descriptor_ui | D064792 |
| mesh[35].is_major_topic | False |
| mesh[35].qualifier_name | |
| mesh[35].descriptor_name | Pragmatic Clinical Trials as Topic |
| mesh[36].qualifier_ui | |
| mesh[36].descriptor_ui | D011788 |
| mesh[36].is_major_topic | False |
| mesh[36].qualifier_name | |
| mesh[36].descriptor_name | Quality of Life |
| mesh[37].qualifier_ui | Q000188 |
| mesh[37].descriptor_ui | D018805 |
| mesh[37].is_major_topic | False |
| mesh[37].qualifier_name | drug therapy |
| mesh[37].descriptor_name | Sepsis |
| mesh[38].qualifier_ui | |
| mesh[38].descriptor_ui | D000078142 |
| mesh[38].is_major_topic | False |
| mesh[38].qualifier_name | |
| mesh[38].descriptor_name | Tazobactam |
| mesh[39].qualifier_ui | |
| mesh[39].descriptor_ui | D016896 |
| mesh[39].is_major_topic | False |
| mesh[39].qualifier_name | |
| mesh[39].descriptor_name | Treatment Outcome |
| mesh[40].qualifier_ui | |
| mesh[40].descriptor_ui | D061605 |
| mesh[40].is_major_topic | False |
| mesh[40].qualifier_name | |
| mesh[40].descriptor_name | Administration, Intravenous |
| mesh[41].qualifier_ui | |
| mesh[41].descriptor_ui | D000284 |
| mesh[41].is_major_topic | False |
| mesh[41].qualifier_name | |
| mesh[41].descriptor_name | Administration, Oral |
| mesh[42].qualifier_ui | |
| mesh[42].descriptor_ui | D019980 |
| mesh[42].is_major_topic | False |
| mesh[42].qualifier_name | |
| mesh[42].descriptor_name | Amoxicillin-Potassium Clavulanate Combination |
| mesh[43].qualifier_ui | Q000008 |
| mesh[43].descriptor_ui | D000900 |
| mesh[43].is_major_topic | False |
| mesh[43].qualifier_name | administration & dosage |
| mesh[43].descriptor_name | Anti-Bacterial Agents |
| mesh[44].qualifier_ui | Q000009 |
| mesh[44].descriptor_ui | D000900 |
| mesh[44].is_major_topic | False |
| mesh[44].qualifier_name | adverse effects |
| mesh[44].descriptor_name | Anti-Bacterial Agents |
| mesh[45].qualifier_ui | |
| mesh[45].descriptor_ui | D002939 |
| mesh[45].is_major_topic | False |
| mesh[45].qualifier_name | |
| mesh[45].descriptor_name | Ciprofloxacin |
| mesh[46].qualifier_ui | Q000191 |
| mesh[46].descriptor_ui | D003362 |
| mesh[46].is_major_topic | False |
| mesh[46].qualifier_name | economics |
| mesh[46].descriptor_name | Cost-Benefit Analysis |
| mesh[47].qualifier_ui | |
| mesh[47].descriptor_ui | D004334 |
| mesh[47].is_major_topic | False |
| mesh[47].qualifier_name | |
| mesh[47].descriptor_name | Drug Administration Schedule |
| mesh[48].qualifier_ui | |
| mesh[48].descriptor_ui | D000074099 |
| mesh[48].is_major_topic | False |
| mesh[48].qualifier_name | |
| mesh[48].descriptor_name | Equivalence Trials as Topic |
| mesh[49].qualifier_ui | |
| mesh[49].descriptor_ui | D006801 |
| mesh[49].is_major_topic | False |
| mesh[49].qualifier_name | |
| mesh[49].descriptor_name | Humans |
| type | article |
| title | Early switch from intravenous to oral antibiotic therapy in patients with cancer who have low-risk neutropenic sepsis (the EASI-SWITCH trial): study protocol for a randomised controlled trial |
| biblio.issue | 1 |
| biblio.volume | 21 |
| biblio.last_page | 431 |
| biblio.first_page | 431 |
| grants[0].funder | https://openalex.org/F4320334661 |
| grants[0].award_id | 13/140/05 |
| grants[0].funder_display_name | Health Technology Assessment Programme |
| topics[0].id | https://openalex.org/T12172 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 1.0 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2730 |
| topics[0].subfield.display_name | Oncology |
| topics[0].display_name | Neutropenia and Cancer Infections |
| topics[1].id | https://openalex.org/T12167 |
| topics[1].field.id | https://openalex.org/fields/13 |
| topics[1].field.display_name | Biochemistry, Genetics and Molecular Biology |
| topics[1].score | 0.9979000091552734 |
| topics[1].domain.id | https://openalex.org/domains/1 |
| topics[1].domain.display_name | Life Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/1308 |
| topics[1].subfield.display_name | Clinical Biochemistry |
| topics[1].display_name | Bacterial Identification and Susceptibility Testing |
| topics[2].id | https://openalex.org/T10218 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9965000152587891 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2713 |
| topics[2].subfield.display_name | Epidemiology |
| topics[2].display_name | Sepsis Diagnosis and Treatment |
| funders[0].id | https://openalex.org/F4320334661 |
| funders[0].ror | https://ror.org/0187kwz08 |
| funders[0].display_name | Health Technology Assessment Programme |
| is_xpac | False |
| apc_list.value | 1570 |
| apc_list.currency | GBP |
| apc_list.value_usd | 1925 |
| apc_paid.value | 1570 |
| apc_paid.currency | GBP |
| apc_paid.value_usd | 1925 |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.938970685005188 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C2778384902 |
| concepts[1].level | 2 |
| concepts[1].score | 0.48862653970718384 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q183134 |
| concepts[1].display_name | Sepsis |
| concepts[2].id | https://openalex.org/C177713679 |
| concepts[2].level | 1 |
| concepts[2].score | 0.46331050992012024 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q679690 |
| concepts[2].display_name | Intensive care medicine |
| concepts[3].id | https://openalex.org/C501593827 |
| concepts[3].level | 2 |
| concepts[3].score | 0.45181822776794434 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q12187 |
| concepts[3].display_name | Antibiotics |
| concepts[4].id | https://openalex.org/C168563851 |
| concepts[4].level | 2 |
| concepts[4].score | 0.4413619041442871 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[4].display_name | Randomized controlled trial |
| concepts[5].id | https://openalex.org/C121608353 |
| concepts[5].level | 2 |
| concepts[5].score | 0.43859827518463135 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[5].display_name | Cancer |
| concepts[6].id | https://openalex.org/C126322002 |
| concepts[6].level | 1 |
| concepts[6].score | 0.37898799777030945 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[6].display_name | Internal medicine |
| concepts[7].id | https://openalex.org/C86803240 |
| concepts[7].level | 0 |
| concepts[7].score | 0.0 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q420 |
| concepts[7].display_name | Biology |
| concepts[8].id | https://openalex.org/C89423630 |
| concepts[8].level | 1 |
| concepts[8].score | 0.0 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q7193 |
| concepts[8].display_name | Microbiology |
| keywords[0].id | https://openalex.org/keywords/medicine |
| keywords[0].score | 0.938970685005188 |
| keywords[0].display_name | Medicine |
| keywords[1].id | https://openalex.org/keywords/sepsis |
| keywords[1].score | 0.48862653970718384 |
| keywords[1].display_name | Sepsis |
| keywords[2].id | https://openalex.org/keywords/intensive-care-medicine |
| keywords[2].score | 0.46331050992012024 |
| keywords[2].display_name | Intensive care medicine |
| keywords[3].id | https://openalex.org/keywords/antibiotics |
| keywords[3].score | 0.45181822776794434 |
| keywords[3].display_name | Antibiotics |
| keywords[4].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[4].score | 0.4413619041442871 |
| keywords[4].display_name | Randomized controlled trial |
| keywords[5].id | https://openalex.org/keywords/cancer |
| keywords[5].score | 0.43859827518463135 |
| keywords[5].display_name | Cancer |
| keywords[6].id | https://openalex.org/keywords/internal-medicine |
| keywords[6].score | 0.37898799777030945 |
| keywords[6].display_name | Internal medicine |
| language | en |
| locations[0].id | doi:10.1186/s13063-020-04241-1 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S109512841 |
| locations[0].source.issn | 1745-6215 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 1745-6215 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Trials |
| locations[0].source.host_organization | https://openalex.org/P4310319900 |
| locations[0].source.host_organization_name | Springer Science+Business Media |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310319900 |
| locations[0].license | cc-by |
| locations[0].pdf_url | https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1 |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Trials |
| locations[0].landing_page_url | https://doi.org/10.1186/s13063-020-04241-1 |
| locations[1].id | pmid:32460818 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Trials |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/32460818 |
| locations[2].id | pmh:oai:https://orca.cardiff.ac.uk:141033 |
| locations[2].is_oa | False |
| locations[2].source.id | https://openalex.org/S4306400871 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | Spectrum Research Repository (Concordia University) |
| locations[2].source.host_organization | https://openalex.org/I60158472 |
| locations[2].source.host_organization_name | Concordia University |
| locations[2].source.host_organization_lineage | https://openalex.org/I60158472 |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | acceptedVersion |
| locations[2].raw_type | Article |
| locations[2].license_id | |
| locations[2].is_accepted | True |
| locations[2].is_published | False |
| locations[2].raw_source_name | |
| locations[2].landing_page_url | |
| locations[3].id | pmh:oai:doaj.org/article:a96bb893c04744328d95f321316c5292 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S4306401280 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[3].source.host_organization | |
| locations[3].source.host_organization_name | |
| locations[3].source.host_organization_lineage | |
| locations[3].license | cc-by-sa |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | article |
| locations[3].license_id | https://openalex.org/licenses/cc-by-sa |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | Trials, Vol 21, Iss 1, Pp 1-11 (2020) |
| locations[3].landing_page_url | https://doaj.org/article/a96bb893c04744328d95f321316c5292 |
| locations[4].id | pmh:oai:pubmedcentral.nih.gov:7251886 |
| locations[4].is_oa | True |
| locations[4].source.id | https://openalex.org/S2764455111 |
| locations[4].source.issn | |
| locations[4].source.type | repository |
| locations[4].source.is_oa | False |
| locations[4].source.issn_l | |
| locations[4].source.is_core | False |
| locations[4].source.is_in_doaj | False |
| locations[4].source.display_name | PubMed Central |
| locations[4].source.host_organization | https://openalex.org/I1299303238 |
| locations[4].source.host_organization_name | National Institutes of Health |
| locations[4].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[4].license | other-oa |
| locations[4].pdf_url | |
| locations[4].version | submittedVersion |
| locations[4].raw_type | Text |
| locations[4].license_id | https://openalex.org/licenses/other-oa |
| locations[4].is_accepted | False |
| locations[4].is_published | False |
| locations[4].raw_source_name | Trials |
| locations[4].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/7251886 |
| indexed_in | crossref, doaj, pubmed |
| authorships[0].author.id | https://openalex.org/A5036465892 |
| authorships[0].author.orcid | https://orcid.org/0000-0001-5021-9973 |
| authorships[0].author.display_name | Caroline Forde |
| authorships[0].countries | GB |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I126231945 |
| authorships[0].affiliations[0].raw_affiliation_string | Centre for Cancer Research and Cell Biology, Queen's University Belfast, Lisburn Road, Belfast, BT9 7AE, UK |
| authorships[0].institutions[0].id | https://openalex.org/I126231945 |
| authorships[0].institutions[0].ror | https://ror.org/00hswnk62 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I126231945 |
| authorships[0].institutions[0].country_code | GB |
| authorships[0].institutions[0].display_name | Queen's University Belfast |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Caroline Forde |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Centre for Cancer Research and Cell Biology, Queen's University Belfast, Lisburn Road, Belfast, BT9 7AE, UK |
| authorships[1].author.id | https://openalex.org/A5025456392 |
| authorships[1].author.orcid | https://orcid.org/0000-0001-6760-6072 |
| authorships[1].author.display_name | Ronan McMullan |
| authorships[1].countries | GB |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I126231945 |
| authorships[1].affiliations[0].raw_affiliation_string | Centre for Experimental Medicine, Queen's University Belfast, Belfast, UK |
| authorships[1].institutions[0].id | https://openalex.org/I126231945 |
| authorships[1].institutions[0].ror | https://ror.org/00hswnk62 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I126231945 |
| authorships[1].institutions[0].country_code | GB |
| authorships[1].institutions[0].display_name | Queen's University Belfast |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Ronan McMullan |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Centre for Experimental Medicine, Queen's University Belfast, Belfast, UK |
| authorships[2].author.id | https://openalex.org/A5068789733 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-2926-7257 |
| authorships[2].author.display_name | Mike Clarke |
| authorships[2].countries | GB |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I126231945 |
| authorships[2].affiliations[0].raw_affiliation_string | Northern Ireland Methodology Hub, Queen's University Belfast, Belfast, UK |
| authorships[2].institutions[0].id | https://openalex.org/I126231945 |
| authorships[2].institutions[0].ror | https://ror.org/00hswnk62 |
| authorships[2].institutions[0].type | education |
| authorships[2].institutions[0].lineage | https://openalex.org/I126231945 |
| authorships[2].institutions[0].country_code | GB |
| authorships[2].institutions[0].display_name | Queen's University Belfast |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Mike Clarke |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Northern Ireland Methodology Hub, Queen's University Belfast, Belfast, UK |
| authorships[3].author.id | https://openalex.org/A5050254542 |
| authorships[3].author.orcid | https://orcid.org/0000-0001-8018-7730 |
| authorships[3].author.display_name | Richard H. Wilson |
| authorships[3].countries | GB |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I7882870 |
| authorships[3].affiliations[0].raw_affiliation_string | Translational Research Centre, University of Glasgow, Glasgow, UK |
| authorships[3].institutions[0].id | https://openalex.org/I7882870 |
| authorships[3].institutions[0].ror | https://ror.org/00vtgdb53 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I7882870 |
| authorships[3].institutions[0].country_code | GB |
| authorships[3].institutions[0].display_name | University of Glasgow |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Richard H. Wilson |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Translational Research Centre, University of Glasgow, Glasgow, UK |
| authorships[4].author.id | https://openalex.org/A5048419838 |
| authorships[4].author.orcid | https://orcid.org/0000-0003-0107-1444 |
| authorships[4].author.display_name | Ruth Plummer |
| authorships[4].countries | GB |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I84884186 |
| authorships[4].affiliations[0].raw_affiliation_string | Northern Institute for Cancer Research, Newcastle University, Newcastle, UK |
| authorships[4].institutions[0].id | https://openalex.org/I84884186 |
| authorships[4].institutions[0].ror | https://ror.org/01kj2bm70 |
| authorships[4].institutions[0].type | education |
| authorships[4].institutions[0].lineage | https://openalex.org/I84884186 |
| authorships[4].institutions[0].country_code | GB |
| authorships[4].institutions[0].display_name | Newcastle University |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Ruth Plummer |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Northern Institute for Cancer Research, Newcastle University, Newcastle, UK |
| authorships[5].author.id | https://openalex.org/A5044432880 |
| authorships[5].author.orcid | https://orcid.org/0000-0002-1861-8541 |
| authorships[5].author.display_name | Margaret Grayson |
| authorships[5].countries | GB |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I4210125479 |
| authorships[5].affiliations[0].raw_affiliation_string | Northern Ireland Cancer Research Consumer Forum, Belfast, UK |
| authorships[5].institutions[0].id | https://openalex.org/I4210125479 |
| authorships[5].institutions[0].ror | https://ror.org/030xykx52 |
| authorships[5].institutions[0].type | healthcare |
| authorships[5].institutions[0].lineage | https://openalex.org/I1289110261, https://openalex.org/I4210125479 |
| authorships[5].institutions[0].country_code | GB |
| authorships[5].institutions[0].display_name | The Northern Ireland Cancer Centre |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Margaret Grayson |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Northern Ireland Cancer Research Consumer Forum, Belfast, UK |
| authorships[6].author.id | https://openalex.org/A5004470262 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-7644-7197 |
| authorships[6].author.display_name | Clíona McDowell |
| authorships[6].countries | GB |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I1289110261 |
| authorships[6].affiliations[0].raw_affiliation_string | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[6].institutions[0].id | https://openalex.org/I1289110261 |
| authorships[6].institutions[0].ror | https://ror.org/02tdmfk69 |
| authorships[6].institutions[0].type | healthcare |
| authorships[6].institutions[0].lineage | https://openalex.org/I1289110261 |
| authorships[6].institutions[0].country_code | GB |
| authorships[6].institutions[0].display_name | Belfast Health and Social Care Trust |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Cliona McDowell |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[7].author.id | https://openalex.org/A5090239373 |
| authorships[7].author.orcid | https://orcid.org/0000-0001-9839-6282 |
| authorships[7].author.display_name | Ashley Agus |
| authorships[7].countries | GB |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I1289110261 |
| authorships[7].affiliations[0].raw_affiliation_string | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[7].institutions[0].id | https://openalex.org/I1289110261 |
| authorships[7].institutions[0].ror | https://ror.org/02tdmfk69 |
| authorships[7].institutions[0].type | healthcare |
| authorships[7].institutions[0].lineage | https://openalex.org/I1289110261 |
| authorships[7].institutions[0].country_code | GB |
| authorships[7].institutions[0].display_name | Belfast Health and Social Care Trust |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Ashley Agus |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[8].author.id | https://openalex.org/A5034187607 |
| authorships[8].author.orcid | https://orcid.org/0000-0002-9802-008X |
| authorships[8].author.display_name | Annmarie Doran |
| authorships[8].countries | GB |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I1289110261 |
| authorships[8].affiliations[0].raw_affiliation_string | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[8].institutions[0].id | https://openalex.org/I1289110261 |
| authorships[8].institutions[0].ror | https://ror.org/02tdmfk69 |
| authorships[8].institutions[0].type | healthcare |
| authorships[8].institutions[0].lineage | https://openalex.org/I1289110261 |
| authorships[8].institutions[0].country_code | GB |
| authorships[8].institutions[0].display_name | Belfast Health and Social Care Trust |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Annmarie Doran |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Northern Ireland Clinical Trials Unit, Belfast Health and Social Care Trust, Belfast, UK |
| authorships[9].author.id | https://openalex.org/A5041103166 |
| authorships[9].author.orcid | https://orcid.org/0000-0002-3283-1947 |
| authorships[9].author.display_name | Daniel F. McAuley |
| authorships[9].countries | GB |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I126231945 |
| authorships[9].affiliations[0].raw_affiliation_string | The Wellcome Wolfson Institute for Experimental Medicine, Queens University Belfast, Belfast, UK |
| authorships[9].institutions[0].id | https://openalex.org/I126231945 |
| authorships[9].institutions[0].ror | https://ror.org/00hswnk62 |
| authorships[9].institutions[0].type | education |
| authorships[9].institutions[0].lineage | https://openalex.org/I126231945 |
| authorships[9].institutions[0].country_code | GB |
| authorships[9].institutions[0].display_name | Queen's University Belfast |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Danny F. McAuley |
| authorships[9].is_corresponding | False |
| authorships[9].raw_affiliation_strings | The Wellcome Wolfson Institute for Experimental Medicine, Queens University Belfast, Belfast, UK |
| authorships[10].author.id | https://openalex.org/A5060639360 |
| authorships[10].author.orcid | https://orcid.org/0000-0003-1998-3134 |
| authorships[10].author.display_name | Anne Thomas |
| authorships[10].countries | GB |
| authorships[10].affiliations[0].institution_ids | https://openalex.org/I4210166781 |
| authorships[10].affiliations[0].raw_affiliation_string | Leicester Cancer Research Centre, Leicester, UK |
| authorships[10].institutions[0].id | https://openalex.org/I4210166781 |
| authorships[10].institutions[0].ror | https://ror.org/05xqxa525 |
| authorships[10].institutions[0].type | facility |
| authorships[10].institutions[0].lineage | https://openalex.org/I143804889, https://openalex.org/I153648349, https://openalex.org/I2802445252, https://openalex.org/I34931013, https://openalex.org/I4210166781 |
| authorships[10].institutions[0].country_code | GB |
| authorships[10].institutions[0].display_name | NIHR Leicester Biomedical Research Centre |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Anne L. Thomas |
| authorships[10].is_corresponding | False |
| authorships[10].raw_affiliation_strings | Leicester Cancer Research Centre, Leicester, UK |
| authorships[11].author.id | https://openalex.org/A5011476580 |
| authorships[11].author.orcid | https://orcid.org/0000-0002-0574-8670 |
| authorships[11].author.display_name | Rosemary A. Barnes |
| authorships[11].countries | GB |
| authorships[11].affiliations[0].institution_ids | https://openalex.org/I79510175 |
| authorships[11].affiliations[0].raw_affiliation_string | Cardiff University School of Medicine, Cardiff, UK |
| authorships[11].institutions[0].id | https://openalex.org/I79510175 |
| authorships[11].institutions[0].ror | https://ror.org/03kk7td41 |
| authorships[11].institutions[0].type | education |
| authorships[11].institutions[0].lineage | https://openalex.org/I79510175 |
| authorships[11].institutions[0].country_code | GB |
| authorships[11].institutions[0].display_name | Cardiff University |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Rosemary A. Barnes |
| authorships[11].is_corresponding | False |
| authorships[11].raw_affiliation_strings | Cardiff University School of Medicine, Cardiff, UK |
| authorships[12].author.id | https://openalex.org/A5017956085 |
| authorships[12].author.orcid | https://orcid.org/0000-0003-3915-7243 |
| authorships[12].author.display_name | Richard Adams |
| authorships[12].countries | GB |
| authorships[12].affiliations[0].institution_ids | https://openalex.org/I2799279586, https://openalex.org/I79510175 |
| authorships[12].affiliations[0].raw_affiliation_string | Cardiff University and Velindre NHS Trust, Cardiff, UK |
| authorships[12].institutions[0].id | https://openalex.org/I79510175 |
| authorships[12].institutions[0].ror | https://ror.org/03kk7td41 |
| authorships[12].institutions[0].type | education |
| authorships[12].institutions[0].lineage | https://openalex.org/I79510175 |
| authorships[12].institutions[0].country_code | GB |
| authorships[12].institutions[0].display_name | Cardiff University |
| authorships[12].institutions[1].id | https://openalex.org/I2799279586 |
| authorships[12].institutions[1].ror | https://ror.org/05ntqkc30 |
| authorships[12].institutions[1].type | healthcare |
| authorships[12].institutions[1].lineage | https://openalex.org/I2799279586 |
| authorships[12].institutions[1].country_code | GB |
| authorships[12].institutions[1].display_name | Velindre NHS Trust |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | Richard Adams |
| authorships[12].is_corresponding | False |
| authorships[12].raw_affiliation_strings | Cardiff University and Velindre NHS Trust, Cardiff, UK |
| authorships[13].author.id | https://openalex.org/A5025120810 |
| authorships[13].author.orcid | https://orcid.org/0000-0003-0286-8703 |
| authorships[13].author.display_name | Ian Chau |
| authorships[13].countries | GB |
| authorships[13].affiliations[0].institution_ids | https://openalex.org/I1325846038 |
| authorships[13].affiliations[0].raw_affiliation_string | The Royal Marsden NHS Foundation Trust, London, UK |
| authorships[13].institutions[0].id | https://openalex.org/I1325846038 |
| authorships[13].institutions[0].ror | https://ror.org/0008wzh48 |
| authorships[13].institutions[0].type | healthcare |
| authorships[13].institutions[0].lineage | https://openalex.org/I1325846038 |
| authorships[13].institutions[0].country_code | GB |
| authorships[13].institutions[0].display_name | Royal Marsden NHS Foundation Trust |
| authorships[13].author_position | middle |
| authorships[13].raw_author_name | Ian Chau |
| authorships[13].is_corresponding | False |
| authorships[13].raw_affiliation_strings | The Royal Marsden NHS Foundation Trust, London, UK |
| authorships[14].author.id | https://openalex.org/A5064190261 |
| authorships[14].author.orcid | https://orcid.org/0000-0001-9566-4130 |
| authorships[14].author.display_name | Vicky M. Coyle |
| authorships[14].countries | GB |
| authorships[14].affiliations[0].institution_ids | https://openalex.org/I126231945 |
| authorships[14].affiliations[0].raw_affiliation_string | Centre for Cancer Research and Cell Biology, Queen's University Belfast, Lisburn Road, Belfast, BT9 7AE, UK |
| authorships[14].institutions[0].id | https://openalex.org/I126231945 |
| authorships[14].institutions[0].ror | https://ror.org/00hswnk62 |
| authorships[14].institutions[0].type | education |
| authorships[14].institutions[0].lineage | https://openalex.org/I126231945 |
| authorships[14].institutions[0].country_code | GB |
| authorships[14].institutions[0].display_name | Queen's University Belfast |
| authorships[14].author_position | last |
| authorships[14].raw_author_name | Vicky Coyle |
| authorships[14].is_corresponding | False |
| authorships[14].raw_affiliation_strings | Centre for Cancer Research and Cell Biology, Queen's University Belfast, Lisburn Road, Belfast, BT9 7AE, UK |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1 |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Early switch from intravenous to oral antibiotic therapy in patients with cancer who have low-risk neutropenic sepsis (the EASI-SWITCH trial): study protocol for a randomised controlled trial |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T12172 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 1.0 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2730 |
| primary_topic.subfield.display_name | Oncology |
| primary_topic.display_name | Neutropenia and Cancer Infections |
| related_works | https://openalex.org/W2355769538, https://openalex.org/W3215560449, https://openalex.org/W2081816252, https://openalex.org/W3030236844, https://openalex.org/W2015216653, https://openalex.org/W2832459556, https://openalex.org/W4237737355, https://openalex.org/W1520457732, https://openalex.org/W2794721326, https://openalex.org/W2047899975 |
| cited_by_count | 5 |
| counts_by_year[0].year | 2024 |
| counts_by_year[0].cited_by_count | 2 |
| counts_by_year[1].year | 2023 |
| counts_by_year[1].cited_by_count | 2 |
| counts_by_year[2].year | 2021 |
| counts_by_year[2].cited_by_count | 1 |
| locations_count | 5 |
| best_oa_location.id | doi:10.1186/s13063-020-04241-1 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S109512841 |
| best_oa_location.source.issn | 1745-6215 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 1745-6215 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Trials |
| best_oa_location.source.host_organization | https://openalex.org/P4310319900 |
| best_oa_location.source.host_organization_name | Springer Science+Business Media |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310319900 |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1 |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Trials |
| best_oa_location.landing_page_url | https://doi.org/10.1186/s13063-020-04241-1 |
| primary_location.id | doi:10.1186/s13063-020-04241-1 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S109512841 |
| primary_location.source.issn | 1745-6215 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 1745-6215 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Trials |
| primary_location.source.host_organization | https://openalex.org/P4310319900 |
| primary_location.source.host_organization_name | Springer Science+Business Media |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310319900 |
| primary_location.license | cc-by |
| primary_location.pdf_url | https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-020-04241-1 |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Trials |
| primary_location.landing_page_url | https://doi.org/10.1186/s13063-020-04241-1 |
| publication_date | 2020-05-27 |
| publication_year | 2020 |
| referenced_works | https://openalex.org/W2341550006, https://openalex.org/W1608439045, https://openalex.org/W3048696092, https://openalex.org/W2788691011, https://openalex.org/W2036841750, https://openalex.org/W2025147742, https://openalex.org/W1932756735, https://openalex.org/W1847321653, https://openalex.org/W2128913758, https://openalex.org/W2481029616, https://openalex.org/W2166023752, https://openalex.org/W2150741204, https://openalex.org/W1990166011, https://openalex.org/W2070673176, https://openalex.org/W1984864482, https://openalex.org/W1971528872, https://openalex.org/W2094119653, https://openalex.org/W2028766754, https://openalex.org/W2108906598, https://openalex.org/W2108696783, https://openalex.org/W4236805817 |
| referenced_works_count | 21 |
| abstract_inverted_index.. | 341 |
| abstract_inverted_index.1 | 337 |
| abstract_inverted_index.5 | 187 |
| abstract_inverted_index.9 | 136 |
| abstract_inverted_index.a | 5, 82, 124, 228, 270, 280 |
| abstract_inverted_index.h | 37, 159, 179, 249 |
| abstract_inverted_index.10 | 135 |
| abstract_inverted_index.16 | 112 |
| abstract_inverted_index.21 | 148 |
| abstract_inverted_index.24 | 158 |
| abstract_inverted_index.38 | 128 |
| abstract_inverted_index.48 | 36, 201 |
| abstract_inverted_index.72 | 248 |
| abstract_inverted_index.IV | 151 |
| abstract_inverted_index.If | 291 |
| abstract_inverted_index.UK | 16, 64 |
| abstract_inverted_index.as | 41, 233 |
| abstract_inverted_index.at | 185, 199, 208, 235 |
| abstract_inverted_index.h, | 202 |
| abstract_inverted_index.in | 103, 144, 275 |
| abstract_inverted_index.is | 81, 227, 287 |
| abstract_inverted_index.it | 221 |
| abstract_inverted_index.of | 48, 95, 126, 141, 180, 230, 296, 308, 324 |
| abstract_inverted_index.on | 269, 335 |
| abstract_inverted_index.or | 153, 192, 246, 266 |
| abstract_inverted_index.to | 32, 57, 74, 89, 100, 162, 170, 172, 194, 261, 299 |
| abstract_inverted_index.× | 134 |
| abstract_inverted_index.(i) | 169 |
| abstract_inverted_index./L, | 137 |
| abstract_inverted_index.1.0 | 133 |
| abstract_inverted_index.14. | 237 |
| abstract_inverted_index.15% | 271, 281 |
| abstract_inverted_index.1:1 | 167 |
| abstract_inverted_index.230 | 288 |
| abstract_inverted_index.The | 15, 63, 79, 223, 238 |
| abstract_inverted_index.and | 21, 93, 149, 175, 279, 310 |
| abstract_inverted_index.are | 160, 165, 214 |
| abstract_inverted_index.day | 236 |
| abstract_inverted_index.for | 9, 19, 38, 44, 61, 67, 155, 198, 240, 305, 319 |
| abstract_inverted_index.low | 42 |
| abstract_inverted_index.not | 25 |
| abstract_inverted_index.the | 71, 91, 209, 276, 284, 292, 320, 336 |
| abstract_inverted_index.≤ | 132 |
| abstract_inverted_index.≥ | 111, 147 |
| abstract_inverted_index.> | 127 |
| abstract_inverted_index.(ii) | 193 |
| abstract_inverted_index.Care | 22, 143 |
| abstract_inverted_index.July | 338 |
| abstract_inverted_index.aims | 88 |
| abstract_inverted_index.care | 264 |
| abstract_inverted_index.cell | 120 |
| abstract_inverted_index.days | 188 |
| abstract_inverted_index.from | 28, 98, 255 |
| abstract_inverted_index.have | 24, 316 |
| abstract_inverted_index.home | 216 |
| abstract_inverted_index.less | 156 |
| abstract_inverted_index.life | 309 |
| abstract_inverted_index.oral | 33, 101, 173, 300 |
| abstract_inverted_index.rate | 274 |
| abstract_inverted_index.risk | 43 |
| abstract_inverted_index.than | 157 |
| abstract_inverted_index.that | 87 |
| abstract_inverted_index.this | 52, 76, 313 |
| abstract_inverted_index.when | 217 |
| abstract_inverted_index.will | 315 |
| abstract_inverted_index.with | 106, 123, 203, 302, 326 |
| abstract_inverted_index.°C, | 129 |
| abstract_inverted_index.2015. | 339 |
| abstract_inverted_index.Based | 268 |
| abstract_inverted_index.MASCC | 138 |
| abstract_inverted_index.Trial | 330 |
| abstract_inverted_index.about | 50 |
| abstract_inverted_index.after | 250 |
| abstract_inverted_index.being | 206 |
| abstract_inverted_index.count | 131 |
| abstract_inverted_index.deems | 220 |
| abstract_inverted_index.early | 96, 297 |
| abstract_inverted_index.fever | 244 |
| abstract_inverted_index.group | 278 |
| abstract_inverted_index.least | 186, 200 |
| abstract_inverted_index.score | 146 |
| abstract_inverted_index.their | 218 |
| abstract_inverted_index.those | 325 |
| abstract_inverted_index.total | 189 |
| abstract_inverted_index.trial | 73, 80, 86, 293 |
| abstract_inverted_index.using | 58 |
| abstract_inverted_index.would | 53 |
| abstract_inverted_index.(acute | 118 |
| abstract_inverted_index.Health | 20, 68 |
| abstract_inverted_index.cancer | 104 |
| abstract_inverted_index.common | 6 |
| abstract_inverted_index.death. | 267 |
| abstract_inverted_index.either | 168 |
| abstract_inverted_index.funded | 70 |
| abstract_inverted_index.sepsis | 3 |
| abstract_inverted_index.septic | 45 |
| abstract_inverted_index.switch | 171 |
| abstract_inverted_index.tackle | 75 |
| abstract_inverted_index.target | 286 |
| abstract_inverted_index.within | 35, 177 |
| abstract_inverted_index.years, | 113 |
| abstract_inverted_index.12–24 | 178 |
| abstract_inverted_index.Cancer) | 145 |
| abstract_inverted_index.ISRCTN: | 332 |
| abstract_inverted_index.Methods | 78 |
| abstract_inverted_index.achieve | 54 |
| abstract_inverted_index.because | 47 |
| abstract_inverted_index.control | 277 |
| abstract_inverted_index.failure | 242, 273 |
| abstract_inverted_index.finding | 314 |
| abstract_inverted_index.include | 243 |
| abstract_inverted_index.longer. | 62 |
| abstract_inverted_index.margin, | 283 |
| abstract_inverted_index.measure | 226 |
| abstract_inverted_index.ongoing | 204 |
| abstract_inverted_index.outcome | 225 |
| abstract_inverted_index.patient | 306 |
| abstract_inverted_index.primary | 224 |
| abstract_inverted_index.quality | 307 |
| abstract_inverted_index.related | 260 |
| abstract_inverted_index.remains | 4 |
| abstract_inverted_index.routine | 321 |
| abstract_inverted_index.sepsis. | 109, 329 |
| abstract_inverted_index.support | 265 |
| abstract_inverted_index.whether | 51 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.EudraCT: | 342 |
| abstract_inverted_index.National | 17, 65 |
| abstract_inverted_index.Patients | 110, 164, 213 |
| abstract_inverted_index.Research | 69 |
| abstract_inverted_index.assessed | 40, 234 |
| abstract_inverted_index.benefits | 304 |
| abstract_inverted_index.clinical | 92, 322 |
| abstract_inverted_index.continue | 195 |
| abstract_inverted_index.criteria | 239 |
| abstract_inverted_index.critical | 263 |
| abstract_inverted_index.eligible | 161 |
| abstract_inverted_index.failures | 232 |
| abstract_inverted_index.hospital | 258 |
| abstract_inverted_index.low-risk | 107, 327 |
| abstract_inverted_index.outcomes | 56 |
| abstract_inverted_index.patients | 10, 39, 105 |
| abstract_inverted_index.resource | 311 |
| abstract_inverted_index.savings, | 312 |
| abstract_inverted_index.starting | 251 |
| abstract_inverted_index.systemic | 12, 115 |
| abstract_inverted_index.84288963. | 333 |
| abstract_inverted_index.Institute | 18, 66 |
| abstract_inverted_index.composite | 229 |
| abstract_inverted_index.continued | 207 |
| abstract_inverted_index.empirical | 29 |
| abstract_inverted_index.establish | 90 |
| abstract_inverted_index.meropenem | 154 |
| abstract_inverted_index.patients. | 289 |
| abstract_inverted_index.physician | 219 |
| abstract_inverted_index.potential | 303 |
| abstract_inverted_index.receiving | 11, 114, 150 |
| abstract_inverted_index.switching | 27, 97, 298 |
| abstract_inverted_index.treatment | 7, 117, 231, 241, 272 |
| abstract_inverted_index.(control). | 212 |
| abstract_inverted_index.Background | 1 |
| abstract_inverted_index.Discussion | 290 |
| abstract_inverted_index.Excellence | 23 |
| abstract_inverted_index.Registered | 334 |
| abstract_inverted_index.Supportive | 142 |
| abstract_inverted_index.commencing | 181 |
| abstract_inverted_index.comparable | 55 |
| abstract_inverted_index.completing | 184 |
| abstract_inverted_index.discharged | 215 |
| abstract_inverted_index.discretion | 211 |
| abstract_inverted_index.escalation | 254 |
| abstract_inverted_index.excluded), | 122 |
| abstract_inverted_index.management | 323 |
| abstract_inverted_index.neutrophil | 130 |
| abstract_inverted_index.pragmatic, | 83 |
| abstract_inverted_index.randomised | 166 |
| abstract_inverted_index.recurrence | 247 |
| abstract_inverted_index.treatment. | 14 |
| abstract_inverted_index.Association | 140 |
| abstract_inverted_index.EASI-SWITCH | 72 |
| abstract_inverted_index.Neutropenic | 2 |
| abstract_inverted_index.anti-cancer | 13, 116 |
| abstract_inverted_index.antibiotics | 31, 34, 60, 102, 190, 197, 205 |
| abstract_inverted_index.intravenous | 30, 59, 99, 182, 196, 252 |
| abstract_inverted_index.neutropenic | 108, 328 |
| abstract_inverted_index.persistence | 245 |
| abstract_inverted_index.randomised, | 84 |
| abstract_inverted_index.readmission | 259 |
| abstract_inverted_index.recommended | 26 |
| abstract_inverted_index.recruitment | 285 |
| abstract_inverted_index.significant | 317 |
| abstract_inverted_index.temperature | 125 |
| abstract_inverted_index.transplants | 121 |
| abstract_inverted_index.uncertainty | 49 |
| abstract_inverted_index.antibiotics, | 183, 253, 257, 301 |
| abstract_inverted_index.appropriate. | 222 |
| abstract_inverted_index.co-amoxiclav | 176 |
| abstract_inverted_index.complication | 8 |
| abstract_inverted_index.demonstrates | 294 |
| abstract_inverted_index.implications | 318 |
| abstract_inverted_index.participate. | 163 |
| abstract_inverted_index.protocolised | 256 |
| abstract_inverted_index.registration | 331 |
| abstract_inverted_index.uncertainty. | 77 |
| abstract_inverted_index.ciprofloxacin | 174 |
| abstract_inverted_index.complications | 46 |
| abstract_inverted_index.physician’s | 210 |
| abstract_inverted_index.(Multinational | 139 |
| abstract_inverted_index.(intervention), | 191 |
| abstract_inverted_index.2015-002830-35. | 343 |
| abstract_inverted_index.leukaemics/stem | 119 |
| abstract_inverted_index.non-inferiority | 85, 282, 295 |
| abstract_inverted_index.cost-effectiveness | 94 |
| abstract_inverted_index.10.1186/ISRCTN84288963 | 340 |
| abstract_inverted_index.infection/antibiotics, | 262 |
| abstract_inverted_index.piperacillin/tazobactam | 152 |
| cited_by_percentile_year.max | 96 |
| cited_by_percentile_year.min | 89 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 15 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.7400000095367432 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.57056334 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |