Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial Article Swipe
 YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.2337/figshare.12245516.v1
YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.2337/figshare.12245516.v1
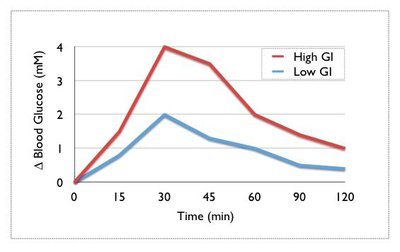
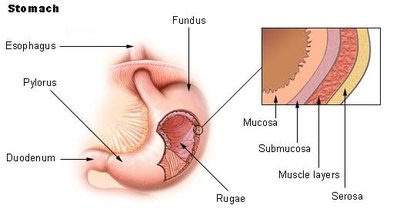
Objective Slowing of gastric emptying by GLP-1 exhibits tachyphylaxis with continuous exposure. We therefore aimed to establish whether prolonged use of a “short-acting” GLP-1 receptor agonist (GLP-1RA), lixisenatide, achieves sustained slowing of gastric emptying and reduction in postprandial glycemia. Research design and methods 30 patients with metformin-treated type 2 diabetes underwent assessment of gastric emptying (scintigraphy) and glucose metabolism (dual tracer technique) after a 75g glucose drink, before and after 8 weeks’ treatment with lixisenatide (20µg subcutaneously daily) or placebo, in a double-blind randomized parallel design. Results Gastric retention of the glucose drink was markedly increased after lixisenatide versus placebo (ratio of adjusted geometric means for area under curve (AUC) over 240 min of 2.19 (95% CI 1.82, 2.64; P<0.001), associated with substantial reductions in the rate of systemic appearance of oral glucose (P<0.001) and incremental AUC for blood glucose (P<0.001). Lixisenatide suppressed both glucagon (P=0.003) and insulin (P=0.032), but not endogenous glucose production, over 120 min after oral glucose. Postprandial glucose-lowering over 240 min was strongly related to the magnitude of slowing of gastric emptying by lixisenatide (r = -0.74, P = 0.002) and to the baseline rate of emptying (r = 0.52, P = 0.048), but unrelated to ß-cell function (assessed by ß-cell glucose sensitivity). Conclusions 8 weeks’ treatment with lixisenatide is associated with sustained slowing of gastric emptying and marked reductions in postprandial glycemia and appearance of ingested glucose. Short-acting GLP-1RAs therefore potentially represent an effective long-term therapy for specifically targeting postprandial glucose excursions.
Related Topics
- Type
- preprint
- Language
- en
- Landing Page
- https://doi.org/10.2337/figshare.12245516.v1
- OA Status
- gold
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4237706364
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4237706364Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.2337/figshare.12245516.v1Digital Object Identifier
- Title
-
Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trialWork title
- Type
-
preprintOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2020Year of publication
- Publication date
-
2020-05-29Full publication date if available
- Authors
-
Christopher K. Rayner, Linda E. Watson, Anna C. Phillips, Kylie Lange, Michelle J. Bound, Jacqueline Grivell, Tongzhi Wu, Karen L. Jones, Michael Horowitz, Ele Ferrannini, Domenico Tricò, Silvia Frascerra, Andrea Mari, Andrea NataliList of authors in order
- Landing page
-
https://doi.org/10.2337/figshare.12245516.v1Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.2337/figshare.12245516.v1Direct OA link when available
- Concepts
-
Lixisenatide, Postprandial, Gastric emptying, Internal medicine, Medicine, Endocrinology, Type 2 diabetes, Metformin, Glucagon, Placebo, Insulin, Crossover study, Diabetes mellitus, Stomach, Exenatide, Alternative medicine, PathologyTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4237706364 |
|---|---|
| doi | https://doi.org/10.2337/figshare.12245516.v1 |
| ids.doi | https://doi.org/10.2337/figshare.12245516.v1 |
| ids.openalex | https://openalex.org/W4237706364 |
| fwci | 0.0 |
| type | preprint |
| title | Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | |
| biblio.first_page | |
| topics[0].id | https://openalex.org/T10401 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9983999729156494 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2712 |
| topics[0].subfield.display_name | Endocrinology, Diabetes and Metabolism |
| topics[0].display_name | Diabetes Treatment and Management |
| topics[1].id | https://openalex.org/T12539 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9962999820709229 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2736 |
| topics[1].subfield.display_name | Pharmacology |
| topics[1].display_name | Pharmacology and Obesity Treatment |
| topics[2].id | https://openalex.org/T10365 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9848999977111816 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2715 |
| topics[2].subfield.display_name | Gastroenterology |
| topics[2].display_name | Gastrointestinal motility and disorders |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C2776442814 |
| concepts[0].level | 5 |
| concepts[0].score | 0.9304512739181519 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q6659956 |
| concepts[0].display_name | Lixisenatide |
| concepts[1].id | https://openalex.org/C2778199505 |
| concepts[1].level | 3 |
| concepts[1].score | 0.8990055322647095 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q2992990 |
| concepts[1].display_name | Postprandial |
| concepts[2].id | https://openalex.org/C3020479747 |
| concepts[2].level | 3 |
| concepts[2].score | 0.8171452283859253 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q1029907 |
| concepts[2].display_name | Gastric emptying |
| concepts[3].id | https://openalex.org/C126322002 |
| concepts[3].level | 1 |
| concepts[3].score | 0.7686355113983154 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[3].display_name | Internal medicine |
| concepts[4].id | https://openalex.org/C71924100 |
| concepts[4].level | 0 |
| concepts[4].score | 0.6782805323600769 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[4].display_name | Medicine |
| concepts[5].id | https://openalex.org/C134018914 |
| concepts[5].level | 1 |
| concepts[5].score | 0.6599500179290771 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q162606 |
| concepts[5].display_name | Endocrinology |
| concepts[6].id | https://openalex.org/C2777180221 |
| concepts[6].level | 3 |
| concepts[6].score | 0.6036546230316162 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q3025883 |
| concepts[6].display_name | Type 2 diabetes |
| concepts[7].id | https://openalex.org/C2780323712 |
| concepts[7].level | 3 |
| concepts[7].score | 0.5508473515510559 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q19484 |
| concepts[7].display_name | Metformin |
| concepts[8].id | https://openalex.org/C2778563252 |
| concepts[8].level | 3 |
| concepts[8].score | 0.500657320022583 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q170617 |
| concepts[8].display_name | Glucagon |
| concepts[9].id | https://openalex.org/C27081682 |
| concepts[9].level | 3 |
| concepts[9].score | 0.4818088412284851 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q269829 |
| concepts[9].display_name | Placebo |
| concepts[10].id | https://openalex.org/C2779306644 |
| concepts[10].level | 2 |
| concepts[10].score | 0.4678070545196533 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q2002370 |
| concepts[10].display_name | Insulin |
| concepts[11].id | https://openalex.org/C87813604 |
| concepts[11].level | 4 |
| concepts[11].score | 0.42611610889434814 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q1242899 |
| concepts[11].display_name | Crossover study |
| concepts[12].id | https://openalex.org/C555293320 |
| concepts[12].level | 2 |
| concepts[12].score | 0.35560595989227295 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q12206 |
| concepts[12].display_name | Diabetes mellitus |
| concepts[13].id | https://openalex.org/C2779422922 |
| concepts[13].level | 2 |
| concepts[13].score | 0.14714741706848145 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q1029907 |
| concepts[13].display_name | Stomach |
| concepts[14].id | https://openalex.org/C2780533449 |
| concepts[14].level | 4 |
| concepts[14].score | 0.13277247548103333 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q417762 |
| concepts[14].display_name | Exenatide |
| concepts[15].id | https://openalex.org/C204787440 |
| concepts[15].level | 2 |
| concepts[15].score | 0.0 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q188504 |
| concepts[15].display_name | Alternative medicine |
| concepts[16].id | https://openalex.org/C142724271 |
| concepts[16].level | 1 |
| concepts[16].score | 0.0 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q7208 |
| concepts[16].display_name | Pathology |
| keywords[0].id | https://openalex.org/keywords/lixisenatide |
| keywords[0].score | 0.9304512739181519 |
| keywords[0].display_name | Lixisenatide |
| keywords[1].id | https://openalex.org/keywords/postprandial |
| keywords[1].score | 0.8990055322647095 |
| keywords[1].display_name | Postprandial |
| keywords[2].id | https://openalex.org/keywords/gastric-emptying |
| keywords[2].score | 0.8171452283859253 |
| keywords[2].display_name | Gastric emptying |
| keywords[3].id | https://openalex.org/keywords/internal-medicine |
| keywords[3].score | 0.7686355113983154 |
| keywords[3].display_name | Internal medicine |
| keywords[4].id | https://openalex.org/keywords/medicine |
| keywords[4].score | 0.6782805323600769 |
| keywords[4].display_name | Medicine |
| keywords[5].id | https://openalex.org/keywords/endocrinology |
| keywords[5].score | 0.6599500179290771 |
| keywords[5].display_name | Endocrinology |
| keywords[6].id | https://openalex.org/keywords/type-2-diabetes |
| keywords[6].score | 0.6036546230316162 |
| keywords[6].display_name | Type 2 diabetes |
| keywords[7].id | https://openalex.org/keywords/metformin |
| keywords[7].score | 0.5508473515510559 |
| keywords[7].display_name | Metformin |
| keywords[8].id | https://openalex.org/keywords/glucagon |
| keywords[8].score | 0.500657320022583 |
| keywords[8].display_name | Glucagon |
| keywords[9].id | https://openalex.org/keywords/placebo |
| keywords[9].score | 0.4818088412284851 |
| keywords[9].display_name | Placebo |
| keywords[10].id | https://openalex.org/keywords/insulin |
| keywords[10].score | 0.4678070545196533 |
| keywords[10].display_name | Insulin |
| keywords[11].id | https://openalex.org/keywords/crossover-study |
| keywords[11].score | 0.42611610889434814 |
| keywords[11].display_name | Crossover study |
| keywords[12].id | https://openalex.org/keywords/diabetes-mellitus |
| keywords[12].score | 0.35560595989227295 |
| keywords[12].display_name | Diabetes mellitus |
| keywords[13].id | https://openalex.org/keywords/stomach |
| keywords[13].score | 0.14714741706848145 |
| keywords[13].display_name | Stomach |
| keywords[14].id | https://openalex.org/keywords/exenatide |
| keywords[14].score | 0.13277247548103333 |
| keywords[14].display_name | Exenatide |
| language | en |
| locations[0].id | doi:10.2337/figshare.12245516.v1 |
| locations[0].is_oa | True |
| locations[0].source | |
| locations[0].license | cc-by-nc-sa |
| locations[0].pdf_url | |
| locations[0].version | acceptedVersion |
| locations[0].raw_type | posted-content |
| locations[0].license_id | https://openalex.org/licenses/cc-by-nc-sa |
| locations[0].is_accepted | True |
| locations[0].is_published | False |
| locations[0].raw_source_name | |
| locations[0].landing_page_url | https://doi.org/10.2337/figshare.12245516.v1 |
| locations[1].id | pmh:oai:figshare.com:article/12245516 |
| locations[1].is_oa | True |
| locations[1].source.id | https://openalex.org/S4306400572 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | OPAL (Open@LaTrobe) (La Trobe University) |
| locations[1].source.host_organization | https://openalex.org/I196829312 |
| locations[1].source.host_organization_name | La Trobe University |
| locations[1].source.host_organization_lineage | https://openalex.org/I196829312 |
| locations[1].license | cc-by-nc-sa |
| locations[1].pdf_url | |
| locations[1].version | submittedVersion |
| locations[1].raw_type | Image |
| locations[1].license_id | https://openalex.org/licenses/cc-by-nc-sa |
| locations[1].is_accepted | False |
| locations[1].is_published | False |
| locations[1].raw_source_name | |
| locations[1].landing_page_url | https://figshare.com/articles/Effects_of_sustained_treatment_with_lixisenatide_on_gastric_emptying_and_postprandial_glucose_metabolism_in_type_2_diabetes_a_randomized_controlled_trial/12245516 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A5056037142 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-5527-256X |
| authorships[0].author.display_name | Christopher K. Rayner |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Christopher K. Rayner |
| authorships[0].is_corresponding | False |
| authorships[1].author.id | https://openalex.org/A5088879990 |
| authorships[1].author.orcid | https://orcid.org/0000-0001-6098-779X |
| authorships[1].author.display_name | Linda E. Watson |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Linda E. Watson |
| authorships[1].is_corresponding | False |
| authorships[2].author.id | https://openalex.org/A5108004671 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-9066-717X |
| authorships[2].author.display_name | Anna C. Phillips |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Liza K. Phillips |
| authorships[2].is_corresponding | False |
| authorships[3].author.id | https://openalex.org/A5000479308 |
| authorships[3].author.orcid | https://orcid.org/0000-0003-3814-8513 |
| authorships[3].author.display_name | Kylie Lange |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Kylie Lange |
| authorships[3].is_corresponding | False |
| authorships[4].author.id | https://openalex.org/A5113570355 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Michelle J. Bound |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Michelle J. Bound |
| authorships[4].is_corresponding | False |
| authorships[5].author.id | https://openalex.org/A5083926609 |
| authorships[5].author.orcid | |
| authorships[5].author.display_name | Jacqueline Grivell |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Jacqueline Grivell |
| authorships[5].is_corresponding | False |
| authorships[6].author.id | https://openalex.org/A5074396199 |
| authorships[6].author.orcid | https://orcid.org/0000-0003-1656-9210 |
| authorships[6].author.display_name | Tongzhi Wu |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Tongzhi Wu |
| authorships[6].is_corresponding | False |
| authorships[7].author.id | https://openalex.org/A5048913465 |
| authorships[7].author.orcid | https://orcid.org/0000-0002-1155-5816 |
| authorships[7].author.display_name | Karen L. Jones |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Karen L. Jones |
| authorships[7].is_corresponding | False |
| authorships[8].author.id | https://openalex.org/A5069463167 |
| authorships[8].author.orcid | https://orcid.org/0000-0002-0942-0306 |
| authorships[8].author.display_name | Michael Horowitz |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Michael Horowitz |
| authorships[8].is_corresponding | False |
| authorships[9].author.id | https://openalex.org/A5089847244 |
| authorships[9].author.orcid | https://orcid.org/0000-0002-1384-1584 |
| authorships[9].author.display_name | Ele Ferrannini |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Ele Ferrannini |
| authorships[9].is_corresponding | False |
| authorships[10].author.id | https://openalex.org/A5024359799 |
| authorships[10].author.orcid | https://orcid.org/0000-0002-7633-1346 |
| authorships[10].author.display_name | Domenico Tricò |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Domenico Tricò |
| authorships[10].is_corresponding | False |
| authorships[11].author.id | https://openalex.org/A5020002476 |
| authorships[11].author.orcid | |
| authorships[11].author.display_name | Silvia Frascerra |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Silvia Frascerra |
| authorships[11].is_corresponding | False |
| authorships[12].author.id | https://openalex.org/A5102028274 |
| authorships[12].author.orcid | https://orcid.org/0000-0002-1436-5591 |
| authorships[12].author.display_name | Andrea Mari |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | Andrea Mari |
| authorships[12].is_corresponding | False |
| authorships[13].author.id | https://openalex.org/A5055458112 |
| authorships[13].author.orcid | https://orcid.org/0000-0002-8465-7717 |
| authorships[13].author.display_name | Andrea Natali |
| authorships[13].author_position | last |
| authorships[13].raw_author_name | Andrea Natali |
| authorships[13].is_corresponding | False |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.2337/figshare.12245516.v1 |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10401 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9983999729156494 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2712 |
| primary_topic.subfield.display_name | Endocrinology, Diabetes and Metabolism |
| primary_topic.display_name | Diabetes Treatment and Management |
| related_works | https://openalex.org/W2623256389, https://openalex.org/W2940950084, https://openalex.org/W2071395483, https://openalex.org/W2163293377, https://openalex.org/W2016633913, https://openalex.org/W4245333134, https://openalex.org/W2106237878, https://openalex.org/W2161051960, https://openalex.org/W2057181942, https://openalex.org/W4288034037 |
| cited_by_count | 0 |
| locations_count | 2 |
| best_oa_location.id | doi:10.2337/figshare.12245516.v1 |
| best_oa_location.is_oa | True |
| best_oa_location.source | |
| best_oa_location.license | cc-by-nc-sa |
| best_oa_location.pdf_url | |
| best_oa_location.version | acceptedVersion |
| best_oa_location.raw_type | posted-content |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by-nc-sa |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | |
| best_oa_location.landing_page_url | https://doi.org/10.2337/figshare.12245516.v1 |
| primary_location.id | doi:10.2337/figshare.12245516.v1 |
| primary_location.is_oa | True |
| primary_location.source | |
| primary_location.license | cc-by-nc-sa |
| primary_location.pdf_url | |
| primary_location.version | acceptedVersion |
| primary_location.raw_type | posted-content |
| primary_location.license_id | https://openalex.org/licenses/cc-by-nc-sa |
| primary_location.is_accepted | True |
| primary_location.is_published | False |
| primary_location.raw_source_name | |
| primary_location.landing_page_url | https://doi.org/10.2337/figshare.12245516.v1 |
| publication_date | 2020-05-29 |
| publication_year | 2020 |
| referenced_works_count | 0 |
| abstract_inverted_index.2 | 51 |
| abstract_inverted_index.8 | 73 |
| abstract_inverted_index.= | 184, 187, 197, 200 |
| abstract_inverted_index.P | 186, 199 |
| abstract_inverted_index.a | 21, 66, 84 |
| abstract_inverted_index.(r | 183, 196 |
| abstract_inverted_index.CI | 121 |
| abstract_inverted_index.We | 12 |
| abstract_inverted_index.an | 244 |
| abstract_inverted_index.by | 5, 181, 208 |
| abstract_inverted_index.in | 36, 83, 129, 231 |
| abstract_inverted_index.is | 220 |
| abstract_inverted_index.of | 2, 20, 31, 55, 94, 106, 118, 132, 135, 176, 178, 194, 225, 236 |
| abstract_inverted_index.or | 81 |
| abstract_inverted_index.to | 15, 173, 190, 204 |
| abstract_inverted_index.120 | 160 |
| abstract_inverted_index.240 | 116, 168 |
| abstract_inverted_index.75g | 67 |
| abstract_inverted_index.AUC | 141 |
| abstract_inverted_index.and | 34, 44, 59, 71, 139, 151, 189, 228, 234 |
| abstract_inverted_index.but | 154, 202 |
| abstract_inverted_index.for | 110, 142, 248 |
| abstract_inverted_index.min | 117, 161, 169 |
| abstract_inverted_index.not | 155 |
| abstract_inverted_index.the | 95, 130, 174, 191 |
| abstract_inverted_index.use | 19 |
| abstract_inverted_index.was | 98, 170 |
| abstract_inverted_index.(95% | 120 |
| abstract_inverted_index.2.19 | 119 |
| abstract_inverted_index.area | 111 |
| abstract_inverted_index.both | 148 |
| abstract_inverted_index.oral | 136, 163 |
| abstract_inverted_index.over | 115, 159, 167 |
| abstract_inverted_index.rate | 131, 193 |
| abstract_inverted_index.type | 50 |
| abstract_inverted_index.with | 9, 48, 76, 126, 218, 222 |
| abstract_inverted_index.(AUC) | 114 |
| abstract_inverted_index.(dual | 62 |
| abstract_inverted_index.0.52, | 198 |
| abstract_inverted_index.1.82, | 122 |
| abstract_inverted_index.2.64; | 123 |
| abstract_inverted_index.GLP-1 | 6, 23 |
| abstract_inverted_index.after | 65, 72, 101, 162 |
| abstract_inverted_index.aimed | 14 |
| abstract_inverted_index.blood | 143 |
| abstract_inverted_index.curve | 113 |
| abstract_inverted_index.drink | 97 |
| abstract_inverted_index.means | 109 |
| abstract_inverted_index.under | 112 |
| abstract_inverted_index.(20µg | 78 |
| abstract_inverted_index.(ratio | 105 |
| abstract_inverted_index.-0.74, | 185 |
| abstract_inverted_index.0.002) | 188 |
| abstract_inverted_index.before | 70 |
| abstract_inverted_index.daily) | 80 |
| abstract_inverted_index.design | 43 |
| abstract_inverted_index.drink, | 69 |
| abstract_inverted_index.marked | 229 |
| abstract_inverted_index.tracer | 63 |
| abstract_inverted_index.versus | 103 |
| abstract_inverted_index.0.048), | 201 |
| abstract_inverted_index.agonist | 25 |
| abstract_inverted_index.gastric | 3, 32, 56, 179, 226 |
| abstract_inverted_index.glucose | 60, 68, 96, 137, 144, 157, 210, 252 |
| abstract_inverted_index.insulin | 152 |
| abstract_inverted_index.placebo | 104 |
| abstract_inverted_index.related | 172 |
| abstract_inverted_index.slowing | 30, 177, 224 |
| abstract_inverted_index.therapy | 247 |
| abstract_inverted_index.whether | 17 |
| abstract_inverted_index.ß-cell | 205, 209 |
| abstract_inverted_index.GLP-1RAs | 240 |
| abstract_inverted_index.achieves | 28 |
| abstract_inverted_index.adjusted | 107 |
| abstract_inverted_index.baseline | 192 |
| abstract_inverted_index.diabetes | 52 |
| abstract_inverted_index.emptying | 4, 33, 57, 180, 195, 227 |
| abstract_inverted_index.exhibits | 7 |
| abstract_inverted_index.function | 206 |
| abstract_inverted_index.glucagon | 149 |
| abstract_inverted_index.glucose. | 164, 238 |
| abstract_inverted_index.glycemia | 233 |
| abstract_inverted_index.ingested | 237 |
| abstract_inverted_index.markedly | 99 |
| abstract_inverted_index.parallel | 87 |
| abstract_inverted_index.patients | 47 |
| abstract_inverted_index.placebo, | 82 |
| abstract_inverted_index.receptor | 24 |
| abstract_inverted_index.strongly | 171 |
| abstract_inverted_index.systemic | 133 |
| abstract_inverted_index.weeks’ | 74, 216 |
| abstract_inverted_index.<p> | 40, 89, 212 |
| abstract_inverted_index.(P=0.003) | 150 |
| abstract_inverted_index.(assessed | 207 |
| abstract_inverted_index.effective | 245 |
| abstract_inverted_index.establish | 16 |
| abstract_inverted_index.exposure. | 11 |
| abstract_inverted_index.geometric | 108 |
| abstract_inverted_index.glycemia. | 38 |
| abstract_inverted_index.increased | 100 |
| abstract_inverted_index.long-term | 246 |
| abstract_inverted_index.magnitude | 175 |
| abstract_inverted_index.prolonged | 18 |
| abstract_inverted_index.reduction | 35 |
| abstract_inverted_index.represent | 243 |
| abstract_inverted_index.retention | 93 |
| abstract_inverted_index.sustained | 29, 223 |
| abstract_inverted_index.targeting | 250 |
| abstract_inverted_index.therefore | 13, 241 |
| abstract_inverted_index.treatment | 75, 217 |
| abstract_inverted_index.underwent | 53 |
| abstract_inverted_index.unrelated | 203 |
| abstract_inverted_index.</p> | 39, 41, 90, 213 |
| abstract_inverted_index.<p>8 | 215 |
| abstract_inverted_index.(GLP-1RA), | 26 |
| abstract_inverted_index.(P=0.032), | 153 |
| abstract_inverted_index.appearance | 134, 235 |
| abstract_inverted_index.assessment | 54 |
| abstract_inverted_index.associated | 125, 221 |
| abstract_inverted_index.continuous | 10 |
| abstract_inverted_index.endogenous | 156 |
| abstract_inverted_index.metabolism | 61 |
| abstract_inverted_index.randomized | 86 |
| abstract_inverted_index.reductions | 128, 230 |
| abstract_inverted_index.suppressed | 147 |
| abstract_inverted_index.technique) | 64 |
| abstract_inverted_index.<p>30 | 46 |
| abstract_inverted_index.incremental | 140 |
| abstract_inverted_index.potentially | 242 |
| abstract_inverted_index.production, | 158 |
| abstract_inverted_index.substantial | 127 |
| abstract_inverted_index.(P<0.001) | 138 |
| abstract_inverted_index.Lixisenatide | 146 |
| abstract_inverted_index.P<0.001), | 124 |
| abstract_inverted_index.Postprandial | 165 |
| abstract_inverted_index.Short-acting | 239 |
| abstract_inverted_index.double-blind | 85 |
| abstract_inverted_index.lixisenatide | 77, 102, 182, 219 |
| abstract_inverted_index.postprandial | 37, 232, 251 |
| abstract_inverted_index.specifically | 249 |
| abstract_inverted_index.(P<0.001). | 145 |
| abstract_inverted_index.lixisenatide, | 27 |
| abstract_inverted_index.tachyphylaxis | 8 |
| abstract_inverted_index.(scintigraphy) | 58 |
| abstract_inverted_index.subcutaneously | 79 |
| abstract_inverted_index.<p>Gastric | 92 |
| abstract_inverted_index.<p>Slowing | 1 |
| abstract_inverted_index.glucose-lowering | 166 |
| abstract_inverted_index.design.</p> | 88 |
| abstract_inverted_index.metformin-treated | 49 |
| abstract_inverted_index.“short-acting” | 22 |
| abstract_inverted_index.excursions.</p> | 253 |
| abstract_inverted_index.sensitivity).</p> | 211 |
| abstract_inverted_index.<p><i>Research | 42 |
| abstract_inverted_index.methods</i></p> | 45 |
| abstract_inverted_index.<i>Objective</i> | 0 |
| abstract_inverted_index.<p><i>Results</i></p> | 91 |
| abstract_inverted_index.<p><i>Conclusions</i></p> | 214 |
| cited_by_percentile_year | |
| countries_distinct_count | 0 |
| institutions_distinct_count | 14 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.5600000023841858 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.32618841 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |