Evaluating the medical management of locally advanced and metastatic basal cell carcinoma: A single institutional retrospective analysis investigating efficacy, safety, and tolerability Article Swipe
 YOU?
·
· 2023
· Open Access
·
· DOI: https://doi.org/10.1016/j.jdin.2023.02.007
YOU?
·
· 2023
· Open Access
·
· DOI: https://doi.org/10.1016/j.jdin.2023.02.007
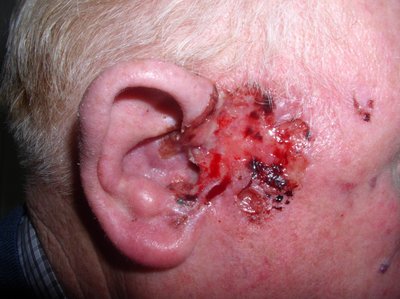
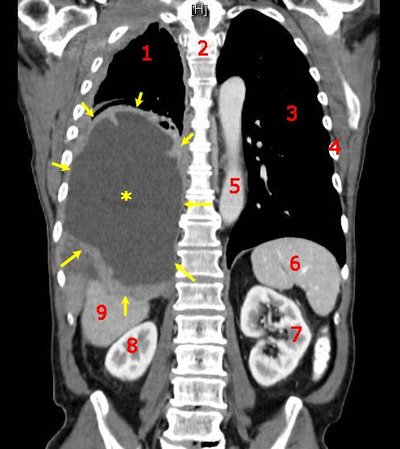
To the Editor: Basal cell carcinoma (BCC) is the most common malignancy globally. Surgery is the first-line treatment. Advanced basal cell carcinoma (aBCC) encompasses metastatic and locally advanced (including large, deep, or aggressive) disease. Metastatic and a subset of locally advanced disease carry a poor prognosis and are generally not amenable to surgery or radiation therapy.1Migden M.R. Chang A.L.S. Dirix L. Stratigos A.J. Lear J.T. Emerging trends in the treatment of advanced basal cell carcinoma.Cancer Treat Rev. 2018; 64: 1-10Abstract Full Text Full Text PDF PubMed Scopus (51) Google Scholar Systemic therapy for aBCC includes hedgehog pathway inhibitors (HPIs), with response rates of 60% but poor tolerability,2Jacobsen A.A. Aldahan A.S. Hughes O.B. Shah V.V. Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies.JAMA Dermatol. 2016; 152: 816-824Crossref PubMed Scopus (74) Google Scholar and immune checkpoint inhibitors (ICIs) (40% response rates).3Chang A.L.S. Tran D.C. Cannon J.G.D. et al.Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study.J Am Acad Dermatol. 2019; 80: 564-566Abstract Full Text Full Text PDF PubMed Scopus (67) Google Scholar,4Stratigos A.J. Sekulic A. Peris K. et al.Primary analysis of phase II results for cemiplimab in patients (pts) with locally advanced basal cell carcinoma (laBCC) who progress on or are intolerant to hedgehog inhibitors (HHIs).Ann Oncol. 2020; 31: S1175-S1176Abstract Full Text Full Text PDF Google Scholar aBCC is uncommon and medical management varies because of the absence of a clear consensus. To investigate our institutional experience treating aBCC, after institutional review board approval we performed a retrospective review of the Yale Hospital electronic medical record (2012-2022). Patients with aBCC treated with vismodegib, sonidegib, nivolumab, ipilimumab, cemiplimab, and/or pembrolizumab were included. Patients with BCC nevus syndrome were excluded. Twenty-three patients (11 metastatic, 12 locally advanced cases) met inclusion criteria (Table I). Prior to systemic treatment, 5 received radiation therapy and 12 had surgery. Thirteen received HPIs (56.5%), 2 received ICIs (8.7%), and 8 received ICIs after HPIs (34.8%).Table IDemographics, clinical characteristics, and oncologic history of patients with advanced basal cell carcinoma treated in this cohortHPI, N = 13∗Median (IQR); n (%).HPI followed by ICI, N = 8∗Median (IQR); n (%).ICI, N = 2∗Median (IQR); n (%).Age at diagnosis69 (63, 75)63 (58, 69)71 (68, 74)Prior skin cancer BCC3 (23.1%)2 (25.0%)0 (0.0%) Melanoma2 (15.4%)2 (25.0%)0 (0.0%) SCC1 (7.7%)0 (0.0%)0 (0.0%) Multiple1 (7.7%)0 (0.0%)0 (0.0%) None6 (46.2%)4 (50.0%)2 (100.0%)Prior therapy†Three patients had repeat surgery for recurrent disease. Radiation2 (15.4%)3 (37.5%)0 (0.0%) Chemotherapy‡One patient had prior chemotherapy treatment with carboplatin and paclitaxel.1 (7.7%)0 (0.0%)0 (0.0%) Excision2 (15.4%)3 (37.5%)0 (0.0%) Mohs6 (46.2%)0 (0.0%)0 (0.0%) Lymph node dissection2 (15.4%)2 (25.0%)0 (0.0%)aBCC subtype Locally advanced9 (69.2%)1 (12.5%)2 (100.0%) Metastatic4 (30.8%)7 (87.5%)0 (0.0%)Metastatic disease Distant3 (23.1%)4 (50.0%)0 (0.0%) Lymph node1 (7.7%)3 (37.5%)0 (0.0%) No metastases9 (69.2%)1 (12.5%)2 (100.0%)aBCC, Advanced basal cell carcinoma; BCC, basal cell carcinoma; HPI, hedgehog pathway inhibitor; ICI, immune checkpoint inhibitor; SCC, squamous cell carcinoma.∗ Median (IQR); n (%).† Three patients had repeat surgery for recurrent disease.‡ One patient had prior chemotherapy treatment with carboplatin and paclitaxel. Open table in a new tab aBCC, Advanced basal cell carcinoma; BCC, basal cell carcinoma; HPI, hedgehog pathway inhibitor; ICI, immune checkpoint inhibitor; SCC, squamous cell carcinoma. Objective response was determined by oncologic documentation according to Response Evaluation Criteria in Solid Tumors criteria, including partial response and complete response. Within the ICI cohort, the objective response rate was 50.0% (20.0% partial response, 30.0% complete response). Objective responses were observed in 52.4% of the HPI cohort (47.6% partial response, 4.8% complete response) (Table II). Of those on systemic treatment alone, 3 of 9 (33.3%) responded to HPIs and 4 of 6 (66.7%) responded to ICIs. Only 23.8% on HPIs and 20.0% on ICIs achieved sustained response after 1 year. Response was not associated with baseline tumor bulk or prior radiation therapy or lymphadenectomy. Patients with metastatic disease were more likely to progress despite treatment (61.1% metastatic, 30.8% locally advanced). At recent follow-up (median time from diagnosis 36.8 months, range 7 to 100 months), disease-specific survival was 72.7% for patients with metastatic and 100% for locally advanced disease.Table IIResponse to medical therapy in patients with advanced basal cell carcinoma treated with hedgehog pathway inhibitors and/or immune checkpoint inhibitors in this cohortHPI, N = 21∗Median (IQR); n (%).ICI, N = 10∗Median (IQR); n (%).Time from diagnosis to treatment initiation (months)6 (2, 72)38 (7, 54)Response to treatment category Overall response11 (52.4%)5 (50.0%)Partial response10 (47.6%)2 (20.0%)Complete response1 (4.8%)3 (30.0%) Progression10 (47.6%)5 (50.0%)Time to response†These values include initial response to treatment in patients who eventually had progression. Less than 3 mo12 (57.1%)6 (60.0%) 3 mo to 1 y5 (23.8%)1 (10.0%) Greater than 1 y1 (4.8%)0 (0.0%) No response1 (4.8%)3 (30.0%) Unknown2 (9.5%)0 (0.0%)Duration of response†These values include initial response to treatment in patients who eventually had progression. Less than 6 mo7 (33.3%)2 (20.0%) 6 mo to 1 y7 (33.3%)3 (30.0%) 1 y to 2 y3 (14.3%)0 (0.0%) Greater than 2 y2 (9.5%)2 (20.0%) No response1 (4.8%)3 (30.0%) Unknown1 (4.8%)0 (0.0%)HPI, Hedgehog pathway inhibitors; ICI, immune checkpoint inhibitors.∗ Median (IQR); n (%).† These values include initial response to treatment in patients who eventually had progression. Open table in a new tab HPI, Hedgehog pathway inhibitors; ICI, immune checkpoint inhibitors. Adverse events (AEs) frequently impacted therapy. Common AEs of HPIs were muscle cramps, dysgeusia, weight loss, and alopecia. Fatigue, diarrhea, and dermatologic toxicities were common with ICIs. Similarly to previous reports,2Jacobsen A.A. Aldahan A.S. Hughes O.B. Shah V.V. Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies.JAMA Dermatol. 2016; 152: 816-824Crossref PubMed Scopus (74) Google Scholar,4Stratigos A.J. Sekulic A. Peris K. et al.Primary analysis of phase II results for cemiplimab in patients (pts) with locally advanced basal cell carcinoma (laBCC) who progress on or are intolerant to hedgehog inhibitors (HHIs).Ann Oncol. 2020; 31: S1175-S1176Abstract Full Text Full Text PDF Google Scholar Grade 3 to 4 AEs resulted in treatment interruption in 38.1% and discontinuation in 33.3% of patients on HPIs (fatigue, muscle cramps, dysgeusia, anorexia), and interruption in 30.0% and discontinuation in 20.0% of patients on ICIs (fatigue, colitis, myocarditis, thyroiditis, adrenal insufficiency). Limitations of our study include its retrospective nature, data from a single institution, small sample size, and variable treatment latency. Nevertheless, we found that overall response rates were in line with previous reports and similar between patients with aBCC who received HPIs and ICIs. The discontinuation rate secondary to AEs of ICIs appeared slightly favorable compared to HPIs. Most patients treated with ICIs first received an HPI. Given that aBCC is rare, a national registry would facilitate further clinical investigation. Dr Leventhal serves on the advisory boards of La Roche-Posay and Sanofi and Regeneron Pharmaceuticals. Dr Burtness served as a consultant for Astra Zeneca, Cue BioPharma, Macrogenics, Vaccinex, ALX Oncology, Arvinas, Nektar, Celgene, Merck, Merck KgA, and Debio Pharm. Dr Christensen has served as a consultant to Sanofi, Regeneron, and LEO pharmaceuticals.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1016/j.jdin.2023.02.007
- http://www.jaadinternational.org/article/S2666328723000342/pdf
- OA Status
- gold
- Cited By
- 2
- References
- 4
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4322102532
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4322102532Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1016/j.jdin.2023.02.007Digital Object Identifier
- Title
-
Evaluating the medical management of locally advanced and metastatic basal cell carcinoma: A single institutional retrospective analysis investigating efficacy, safety, and tolerabilityWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2023Year of publication
- Publication date
-
2023-02-26Full publication date if available
- Authors
-
Annika Belzer, Jolanta Pach, Ryland D. Mortlock, James Clune, Kelly Olino, Mario Sznol, Aarti Bhatia, Barbara Burtness, Sean R. Christensen, Jonathan S. LeventhalList of authors in order
- Landing page
-
https://doi.org/10.1016/j.jdin.2023.02.007Publisher landing page
- PDF URL
-
https://www.jaadinternational.org/article/S2666328723000342/pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://www.jaadinternational.org/article/S2666328723000342/pdfDirect OA link when available
- Concepts
-
Tolerability, Basal cell carcinoma, Medicine, Internal medicine, Oncology, Radiation therapy, Vismodegib, Pembrolizumab, Malignancy, Cancer, Immunotherapy, Adverse effect, Basal cellTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
2Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 1, 2024: 1Per-year citation counts (last 5 years)
- References (count)
-
4Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4322102532 |
|---|---|
| doi | https://doi.org/10.1016/j.jdin.2023.02.007 |
| ids.doi | https://doi.org/10.1016/j.jdin.2023.02.007 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/37252181 |
| ids.openalex | https://openalex.org/W4322102532 |
| fwci | 0.37134082 |
| type | article |
| title | Evaluating the medical management of locally advanced and metastatic basal cell carcinoma: A single institutional retrospective analysis investigating efficacy, safety, and tolerability |
| biblio.issue | |
| biblio.volume | 11 |
| biblio.last_page | 175 |
| biblio.first_page | 174 |
| topics[0].id | https://openalex.org/T12009 |
| topics[0].field.id | https://openalex.org/fields/13 |
| topics[0].field.display_name | Biochemistry, Genetics and Molecular Biology |
| topics[0].score | 0.9998999834060669 |
| topics[0].domain.id | https://openalex.org/domains/1 |
| topics[0].domain.display_name | Life Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/1312 |
| topics[0].subfield.display_name | Molecular Biology |
| topics[0].display_name | Hedgehog Signaling Pathway Studies |
| topics[1].id | https://openalex.org/T11306 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9993000030517578 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2713 |
| topics[1].subfield.display_name | Epidemiology |
| topics[1].display_name | Nonmelanoma Skin Cancer Studies |
| topics[2].id | https://openalex.org/T13383 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9937999844551086 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2734 |
| topics[2].subfield.display_name | Pathology and Forensic Medicine |
| topics[2].display_name | Tumors and Oncological Cases |
| is_xpac | False |
| apc_list.value | 2500 |
| apc_list.currency | USD |
| apc_list.value_usd | 2500 |
| apc_paid.value | 2500 |
| apc_paid.currency | USD |
| apc_paid.value_usd | 2500 |
| concepts[0].id | https://openalex.org/C2778375690 |
| concepts[0].level | 3 |
| concepts[0].score | 0.7559138536453247 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q7814214 |
| concepts[0].display_name | Tolerability |
| concepts[1].id | https://openalex.org/C2778804307 |
| concepts[1].level | 3 |
| concepts[1].score | 0.7051695585250854 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q809758 |
| concepts[1].display_name | Basal cell carcinoma |
| concepts[2].id | https://openalex.org/C71924100 |
| concepts[2].level | 0 |
| concepts[2].score | 0.698594331741333 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[2].display_name | Medicine |
| concepts[3].id | https://openalex.org/C126322002 |
| concepts[3].level | 1 |
| concepts[3].score | 0.48529380559921265 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[3].display_name | Internal medicine |
| concepts[4].id | https://openalex.org/C143998085 |
| concepts[4].level | 1 |
| concepts[4].score | 0.4759792387485504 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q162555 |
| concepts[4].display_name | Oncology |
| concepts[5].id | https://openalex.org/C509974204 |
| concepts[5].level | 2 |
| concepts[5].score | 0.4258677661418915 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q180507 |
| concepts[5].display_name | Radiation therapy |
| concepts[6].id | https://openalex.org/C2779545874 |
| concepts[6].level | 4 |
| concepts[6].score | 0.4225963354110718 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q2070286 |
| concepts[6].display_name | Vismodegib |
| concepts[7].id | https://openalex.org/C2780057760 |
| concepts[7].level | 4 |
| concepts[7].score | 0.41507089138031006 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q13896859 |
| concepts[7].display_name | Pembrolizumab |
| concepts[8].id | https://openalex.org/C2779399171 |
| concepts[8].level | 2 |
| concepts[8].score | 0.41458889842033386 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q1483951 |
| concepts[8].display_name | Malignancy |
| concepts[9].id | https://openalex.org/C121608353 |
| concepts[9].level | 2 |
| concepts[9].score | 0.37014779448509216 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[9].display_name | Cancer |
| concepts[10].id | https://openalex.org/C2777701055 |
| concepts[10].level | 3 |
| concepts[10].score | 0.24545449018478394 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q1427096 |
| concepts[10].display_name | Immunotherapy |
| concepts[11].id | https://openalex.org/C197934379 |
| concepts[11].level | 2 |
| concepts[11].score | 0.19627046585083008 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[11].display_name | Adverse effect |
| concepts[12].id | https://openalex.org/C3019992690 |
| concepts[12].level | 2 |
| concepts[12].score | 0.19101035594940186 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q92767510 |
| concepts[12].display_name | Basal cell |
| keywords[0].id | https://openalex.org/keywords/tolerability |
| keywords[0].score | 0.7559138536453247 |
| keywords[0].display_name | Tolerability |
| keywords[1].id | https://openalex.org/keywords/basal-cell-carcinoma |
| keywords[1].score | 0.7051695585250854 |
| keywords[1].display_name | Basal cell carcinoma |
| keywords[2].id | https://openalex.org/keywords/medicine |
| keywords[2].score | 0.698594331741333 |
| keywords[2].display_name | Medicine |
| keywords[3].id | https://openalex.org/keywords/internal-medicine |
| keywords[3].score | 0.48529380559921265 |
| keywords[3].display_name | Internal medicine |
| keywords[4].id | https://openalex.org/keywords/oncology |
| keywords[4].score | 0.4759792387485504 |
| keywords[4].display_name | Oncology |
| keywords[5].id | https://openalex.org/keywords/radiation-therapy |
| keywords[5].score | 0.4258677661418915 |
| keywords[5].display_name | Radiation therapy |
| keywords[6].id | https://openalex.org/keywords/vismodegib |
| keywords[6].score | 0.4225963354110718 |
| keywords[6].display_name | Vismodegib |
| keywords[7].id | https://openalex.org/keywords/pembrolizumab |
| keywords[7].score | 0.41507089138031006 |
| keywords[7].display_name | Pembrolizumab |
| keywords[8].id | https://openalex.org/keywords/malignancy |
| keywords[8].score | 0.41458889842033386 |
| keywords[8].display_name | Malignancy |
| keywords[9].id | https://openalex.org/keywords/cancer |
| keywords[9].score | 0.37014779448509216 |
| keywords[9].display_name | Cancer |
| keywords[10].id | https://openalex.org/keywords/immunotherapy |
| keywords[10].score | 0.24545449018478394 |
| keywords[10].display_name | Immunotherapy |
| keywords[11].id | https://openalex.org/keywords/adverse-effect |
| keywords[11].score | 0.19627046585083008 |
| keywords[11].display_name | Adverse effect |
| keywords[12].id | https://openalex.org/keywords/basal-cell |
| keywords[12].score | 0.19101035594940186 |
| keywords[12].display_name | Basal cell |
| language | en |
| locations[0].id | doi:10.1016/j.jdin.2023.02.007 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S4210208778 |
| locations[0].source.issn | 2666-3287 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 2666-3287 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | JAAD International |
| locations[0].source.host_organization | https://openalex.org/P4310320990 |
| locations[0].source.host_organization_name | Elsevier BV |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320990 |
| locations[0].license | cc-by-nc-nd |
| locations[0].pdf_url | http://www.jaadinternational.org/article/S2666328723000342/pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by-nc-nd |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | JAAD International |
| locations[0].landing_page_url | https://doi.org/10.1016/j.jdin.2023.02.007 |
| locations[1].id | pmid:37252181 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | JAAD international |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/37252181 |
| locations[2].id | pmh:oai:pubmedcentral.nih.gov:10213716 |
| locations[2].is_oa | True |
| locations[2].source.id | https://openalex.org/S2764455111 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | PubMed Central |
| locations[2].source.host_organization | https://openalex.org/I1299303238 |
| locations[2].source.host_organization_name | National Institutes of Health |
| locations[2].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[2].license | cc-by-nc-nd |
| locations[2].pdf_url | https://pmc.ncbi.nlm.nih.gov/articles/PMC10213716/pdf/main.pdf |
| locations[2].version | submittedVersion |
| locations[2].raw_type | Text |
| locations[2].license_id | https://openalex.org/licenses/cc-by-nc-nd |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | JAAD Int |
| locations[2].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/10213716 |
| locations[3].id | pmh:oai:doaj.org/article:688703ca2dd34aacafe1b31efb879652 |
| locations[3].is_oa | False |
| locations[3].source.id | https://openalex.org/S4306401280 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[3].source.host_organization | |
| locations[3].source.host_organization_name | |
| locations[3].source.host_organization_lineage | |
| locations[3].license | |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | article |
| locations[3].license_id | |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | JAAD International, Vol 11, Iss , Pp 174-175 (2023) |
| locations[3].landing_page_url | https://doaj.org/article/688703ca2dd34aacafe1b31efb879652 |
| indexed_in | crossref, doaj, pubmed |
| authorships[0].author.id | https://openalex.org/A5008630478 |
| authorships[0].author.orcid | https://orcid.org/0000-0003-2228-4107 |
| authorships[0].author.display_name | Annika Belzer |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[0].affiliations[0].raw_affiliation_string | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[0].institutions[0].id | https://openalex.org/I32971472 |
| authorships[0].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | Yale University |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Annika Belzer |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[1].author.id | https://openalex.org/A5019600041 |
| authorships[1].author.orcid | https://orcid.org/0000-0003-2440-7756 |
| authorships[1].author.display_name | Jolanta Pach |
| authorships[1].countries | US |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[1].affiliations[0].raw_affiliation_string | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[1].institutions[0].id | https://openalex.org/I32971472 |
| authorships[1].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[1].institutions[0].country_code | US |
| authorships[1].institutions[0].display_name | Yale University |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Jolanta Pach |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[2].author.id | https://openalex.org/A5042073058 |
| authorships[2].author.orcid | https://orcid.org/0000-0001-9666-4394 |
| authorships[2].author.display_name | Ryland D. Mortlock |
| authorships[2].countries | US |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[2].affiliations[0].raw_affiliation_string | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[2].institutions[0].id | https://openalex.org/I32971472 |
| authorships[2].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[2].institutions[0].type | education |
| authorships[2].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[2].institutions[0].country_code | US |
| authorships[2].institutions[0].display_name | Yale University |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Ryland D. Mortlock |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[3].author.id | https://openalex.org/A5002821731 |
| authorships[3].author.orcid | https://orcid.org/0000-0002-2398-4634 |
| authorships[3].author.display_name | James Clune |
| authorships[3].countries | US |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[3].affiliations[0].raw_affiliation_string | Department of Plastic Surgery, Yale School of Medicine, New Haven, Connecticut |
| authorships[3].institutions[0].id | https://openalex.org/I32971472 |
| authorships[3].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[3].institutions[0].country_code | US |
| authorships[3].institutions[0].display_name | Yale University |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | James Clune |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Department of Plastic Surgery, Yale School of Medicine, New Haven, Connecticut |
| authorships[4].author.id | https://openalex.org/A5021303180 |
| authorships[4].author.orcid | https://orcid.org/0000-0001-6209-5218 |
| authorships[4].author.display_name | Kelly Olino |
| authorships[4].countries | US |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[4].affiliations[0].raw_affiliation_string | Department of Surgical Oncology, Yale School of Medicine, New Haven, Connecticut |
| authorships[4].institutions[0].id | https://openalex.org/I32971472 |
| authorships[4].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[4].institutions[0].type | education |
| authorships[4].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[4].institutions[0].country_code | US |
| authorships[4].institutions[0].display_name | Yale University |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Kelly Olino |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Department of Surgical Oncology, Yale School of Medicine, New Haven, Connecticut |
| authorships[5].author.id | https://openalex.org/A5007198397 |
| authorships[5].author.orcid | https://orcid.org/0000-0002-4359-8749 |
| authorships[5].author.display_name | Mario Sznol |
| authorships[5].countries | US |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I2802730090 |
| authorships[5].affiliations[0].raw_affiliation_string | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[5].institutions[0].id | https://openalex.org/I2802730090 |
| authorships[5].institutions[0].ror | https://ror.org/03j7sze86 |
| authorships[5].institutions[0].type | facility |
| authorships[5].institutions[0].lineage | https://openalex.org/I2802730090, https://openalex.org/I4210117499, https://openalex.org/I4210165192 |
| authorships[5].institutions[0].country_code | US |
| authorships[5].institutions[0].display_name | Yale Cancer Center |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Mario Sznol |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[6].author.id | https://openalex.org/A5078179040 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-0491-4454 |
| authorships[6].author.display_name | Aarti Bhatia |
| authorships[6].countries | US |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I2802730090 |
| authorships[6].affiliations[0].raw_affiliation_string | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[6].institutions[0].id | https://openalex.org/I2802730090 |
| authorships[6].institutions[0].ror | https://ror.org/03j7sze86 |
| authorships[6].institutions[0].type | facility |
| authorships[6].institutions[0].lineage | https://openalex.org/I2802730090, https://openalex.org/I4210117499, https://openalex.org/I4210165192 |
| authorships[6].institutions[0].country_code | US |
| authorships[6].institutions[0].display_name | Yale Cancer Center |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Aarti Bhatia |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[7].author.id | https://openalex.org/A5023000705 |
| authorships[7].author.orcid | https://orcid.org/0000-0003-4660-1859 |
| authorships[7].author.display_name | Barbara Burtness |
| authorships[7].countries | US |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I2802730090 |
| authorships[7].affiliations[0].raw_affiliation_string | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[7].institutions[0].id | https://openalex.org/I2802730090 |
| authorships[7].institutions[0].ror | https://ror.org/03j7sze86 |
| authorships[7].institutions[0].type | facility |
| authorships[7].institutions[0].lineage | https://openalex.org/I2802730090, https://openalex.org/I4210117499, https://openalex.org/I4210165192 |
| authorships[7].institutions[0].country_code | US |
| authorships[7].institutions[0].display_name | Yale Cancer Center |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Barbara Burtness |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Department of Internal Medicine and Yale Cancer Center, Yale School of Medicine, New Haven, Connecticut |
| authorships[8].author.id | https://openalex.org/A5070727750 |
| authorships[8].author.orcid | https://orcid.org/0000-0003-2711-0026 |
| authorships[8].author.display_name | Sean R. Christensen |
| authorships[8].countries | US |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[8].affiliations[0].raw_affiliation_string | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[8].institutions[0].id | https://openalex.org/I32971472 |
| authorships[8].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[8].institutions[0].type | education |
| authorships[8].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[8].institutions[0].country_code | US |
| authorships[8].institutions[0].display_name | Yale University |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Sean Christensen |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[9].author.id | https://openalex.org/A5041289456 |
| authorships[9].author.orcid | https://orcid.org/0000-0002-3757-8477 |
| authorships[9].author.display_name | Jonathan S. Leventhal |
| authorships[9].countries | US |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I32971472 |
| authorships[9].affiliations[0].raw_affiliation_string | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| authorships[9].institutions[0].id | https://openalex.org/I32971472 |
| authorships[9].institutions[0].ror | https://ror.org/03v76x132 |
| authorships[9].institutions[0].type | education |
| authorships[9].institutions[0].lineage | https://openalex.org/I32971472 |
| authorships[9].institutions[0].country_code | US |
| authorships[9].institutions[0].display_name | Yale University |
| authorships[9].author_position | last |
| authorships[9].raw_author_name | Jonathan S. Leventhal |
| authorships[9].is_corresponding | True |
| authorships[9].raw_affiliation_strings | Department of Dermatology, Yale School of Medicine, New Haven, Connecticut |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | http://www.jaadinternational.org/article/S2666328723000342/pdf |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Evaluating the medical management of locally advanced and metastatic basal cell carcinoma: A single institutional retrospective analysis investigating efficacy, safety, and tolerability |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T12009 |
| primary_topic.field.id | https://openalex.org/fields/13 |
| primary_topic.field.display_name | Biochemistry, Genetics and Molecular Biology |
| primary_topic.score | 0.9998999834060669 |
| primary_topic.domain.id | https://openalex.org/domains/1 |
| primary_topic.domain.display_name | Life Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/1312 |
| primary_topic.subfield.display_name | Molecular Biology |
| primary_topic.display_name | Hedgehog Signaling Pathway Studies |
| related_works | https://openalex.org/W1920413731, https://openalex.org/W3126292731, https://openalex.org/W2071250613, https://openalex.org/W1803222855, https://openalex.org/W2153743952, https://openalex.org/W3045377858, https://openalex.org/W2593422178, https://openalex.org/W4304804698, https://openalex.org/W2607360241, https://openalex.org/W2464850765 |
| cited_by_count | 2 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 1 |
| counts_by_year[1].year | 2024 |
| counts_by_year[1].cited_by_count | 1 |
| locations_count | 4 |
| best_oa_location.id | doi:10.1016/j.jdin.2023.02.007 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S4210208778 |
| best_oa_location.source.issn | 2666-3287 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 2666-3287 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | JAAD International |
| best_oa_location.source.host_organization | https://openalex.org/P4310320990 |
| best_oa_location.source.host_organization_name | Elsevier BV |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| best_oa_location.license | cc-by-nc-nd |
| best_oa_location.pdf_url | http://www.jaadinternational.org/article/S2666328723000342/pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by-nc-nd |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | JAAD International |
| best_oa_location.landing_page_url | https://doi.org/10.1016/j.jdin.2023.02.007 |
| primary_location.id | doi:10.1016/j.jdin.2023.02.007 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S4210208778 |
| primary_location.source.issn | 2666-3287 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 2666-3287 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | JAAD International |
| primary_location.source.host_organization | https://openalex.org/P4310320990 |
| primary_location.source.host_organization_name | Elsevier BV |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| primary_location.license | cc-by-nc-nd |
| primary_location.pdf_url | http://www.jaadinternational.org/article/S2666328723000342/pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by-nc-nd |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | JAAD International |
| primary_location.landing_page_url | https://doi.org/10.1016/j.jdin.2023.02.007 |
| publication_date | 2023-02-26 |
| publication_year | 2023 |
| referenced_works | https://openalex.org/W2771198691, https://openalex.org/W2341152357, https://openalex.org/W2888104081, https://openalex.org/W3088124939 |
| referenced_works_count | 4 |
| abstract_inverted_index.1 | 622, 766, 772, 806, 810 |
| abstract_inverted_index.2 | 321, 813, 819 |
| abstract_inverted_index.3 | 595, 759, 763, 984 |
| abstract_inverted_index.4 | 603, 986 |
| abstract_inverted_index.5 | 309 |
| abstract_inverted_index.6 | 605, 799, 803 |
| abstract_inverted_index.7 | 664 |
| abstract_inverted_index.8 | 326 |
| abstract_inverted_index.9 | 597 |
| abstract_inverted_index.= | 350, 359, 365, 706, 712 |
| abstract_inverted_index.N | 349, 358, 364, 705, 711 |
| abstract_inverted_index.a | 36, 43, 128, 243, 260, 509, 857, 920, 1035, 1097, 1124, 1149 |
| abstract_inverted_index.n | 353, 362, 368, 486, 709, 715, 839 |
| abstract_inverted_index.y | 811 |
| abstract_inverted_index.12 | 296, 314 |
| abstract_inverted_index.A. | 188, 940 |
| abstract_inverted_index.Am | 170 |
| abstract_inverted_index.At | 654 |
| abstract_inverted_index.Dr | 1105, 1120, 1144 |
| abstract_inverted_index.II | 196, 948 |
| abstract_inverted_index.J. | 115, 907 |
| abstract_inverted_index.K. | 190, 942 |
| abstract_inverted_index.L. | 60 |
| abstract_inverted_index.La | 1113 |
| abstract_inverted_index.No | 459, 776, 823 |
| abstract_inverted_index.Of | 589 |
| abstract_inverted_index.To | 0, 246 |
| abstract_inverted_index.an | 166, 1090 |
| abstract_inverted_index.as | 1123, 1148 |
| abstract_inverted_index.at | 370 |
| abstract_inverted_index.by | 356, 537 |
| abstract_inverted_index.et | 159, 191, 943 |
| abstract_inverted_index.in | 67, 200, 346, 508, 545, 575, 686, 702, 751, 791, 848, 856, 952, 989, 992, 996, 1009, 1013, 1053 |
| abstract_inverted_index.is | 7, 14, 232, 1095 |
| abstract_inverted_index.mo | 764, 804 |
| abstract_inverted_index.of | 38, 70, 102, 134, 194, 239, 242, 263, 338, 577, 596, 604, 783, 876, 926, 946, 998, 1015, 1026, 1075, 1112 |
| abstract_inverted_index.on | 212, 591, 612, 616, 964, 1000, 1017, 1108 |
| abstract_inverted_index.or | 31, 53, 213, 632, 636, 965 |
| abstract_inverted_index.to | 51, 216, 306, 541, 600, 608, 645, 665, 683, 719, 727, 743, 749, 765, 789, 805, 812, 846, 896, 968, 985, 1073, 1081, 1151 |
| abstract_inverted_index.we | 258, 1046 |
| abstract_inverted_index.y1 | 773 |
| abstract_inverted_index.y2 | 820 |
| abstract_inverted_index.y3 | 814 |
| abstract_inverted_index.y5 | 767 |
| abstract_inverted_index.y7 | 807 |
| abstract_inverted_index.(11 | 294 |
| abstract_inverted_index.(2, | 723 |
| abstract_inverted_index.(7, | 725 |
| abstract_inverted_index.100 | 666 |
| abstract_inverted_index.31: | 222, 974 |
| abstract_inverted_index.60% | 103 |
| abstract_inverted_index.64: | 78 |
| abstract_inverted_index.80: | 174 |
| abstract_inverted_index.AEs | 875, 987, 1074 |
| abstract_inverted_index.ALX | 1133 |
| abstract_inverted_index.BCC | 287 |
| abstract_inverted_index.Cue | 1129 |
| abstract_inverted_index.HPI | 579 |
| abstract_inverted_index.I). | 304 |
| abstract_inverted_index.ICI | 557 |
| abstract_inverted_index.LEO | 1155 |
| abstract_inverted_index.One | 496 |
| abstract_inverted_index.PDF | 84, 180, 228, 980 |
| abstract_inverted_index.The | 1069 |
| abstract_inverted_index.and | 25, 35, 46, 123, 131, 146, 234, 313, 325, 335, 420, 504, 552, 602, 614, 676, 884, 888, 915, 923, 994, 1007, 1011, 1041, 1058, 1067, 1115, 1117, 1141, 1154 |
| abstract_inverted_index.are | 47, 214, 966 |
| abstract_inverted_index.but | 104 |
| abstract_inverted_index.for | 92, 120, 161, 198, 405, 493, 672, 678, 912, 950, 1126 |
| abstract_inverted_index.had | 315, 402, 414, 490, 498, 755, 795, 852 |
| abstract_inverted_index.has | 1146 |
| abstract_inverted_index.its | 1030 |
| abstract_inverted_index.met | 300 |
| abstract_inverted_index.mo7 | 800 |
| abstract_inverted_index.new | 510, 858 |
| abstract_inverted_index.not | 49, 626 |
| abstract_inverted_index.our | 248, 1027 |
| abstract_inverted_index.tab | 511, 859 |
| abstract_inverted_index.the | 1, 8, 15, 68, 240, 264, 556, 559, 578, 1109 |
| abstract_inverted_index.was | 535, 563, 625, 670 |
| abstract_inverted_index.who | 210, 753, 793, 850, 962, 1064 |
| abstract_inverted_index.(40% | 151 |
| abstract_inverted_index.(51) | 87 |
| abstract_inverted_index.(58, | 374 |
| abstract_inverted_index.(63, | 372 |
| abstract_inverted_index.(67) | 183 |
| abstract_inverted_index.(68, | 376 |
| abstract_inverted_index.(74) | 143, 935 |
| abstract_inverted_index.100% | 677 |
| abstract_inverted_index.152: | 139, 931 |
| abstract_inverted_index.36.8 | 661 |
| abstract_inverted_index.4.8% | 584 |
| abstract_inverted_index.A.A. | 107, 899 |
| abstract_inverted_index.A.J. | 62, 186, 938 |
| abstract_inverted_index.A.S. | 109, 901 |
| abstract_inverted_index.Acad | 171 |
| abstract_inverted_index.BCC, | 468, 517 |
| abstract_inverted_index.BCC3 | 380 |
| abstract_inverted_index.D.C. | 156 |
| abstract_inverted_index.Full | 80, 82, 176, 178, 224, 226, 976, 978 |
| abstract_inverted_index.HPI, | 472, 521, 860 |
| abstract_inverted_index.HPI. | 1091 |
| abstract_inverted_index.HPIs | 319, 330, 601, 613, 877, 1001, 1066 |
| abstract_inverted_index.ICI, | 357, 476, 525, 833, 864 |
| abstract_inverted_index.ICIs | 323, 328, 617, 1018, 1076, 1087 |
| abstract_inverted_index.II). | 588 |
| abstract_inverted_index.J.T. | 64 |
| abstract_inverted_index.KgA, | 1140 |
| abstract_inverted_index.Lear | 63 |
| abstract_inverted_index.Less | 757, 797 |
| abstract_inverted_index.M.R. | 56 |
| abstract_inverted_index.Most | 1083 |
| abstract_inverted_index.O.B. | 111, 903 |
| abstract_inverted_index.Only | 610 |
| abstract_inverted_index.Open | 506, 854 |
| abstract_inverted_index.Rev. | 76 |
| abstract_inverted_index.SCC, | 480, 529 |
| abstract_inverted_index.SCC1 | 388 |
| abstract_inverted_index.Shah | 112, 904 |
| abstract_inverted_index.Text | 81, 83, 177, 179, 225, 227, 977, 979 |
| abstract_inverted_index.Tran | 155 |
| abstract_inverted_index.V.V. | 113, 905 |
| abstract_inverted_index.Yale | 265 |
| abstract_inverted_index.aBCC | 93, 231, 273, 1063, 1094 |
| abstract_inverted_index.bulk | 631 |
| abstract_inverted_index.cell | 4, 20, 73, 126, 164, 207, 343, 466, 470, 482, 515, 519, 531, 691, 918, 959 |
| abstract_inverted_index.data | 1033 |
| abstract_inverted_index.from | 659, 717, 1034 |
| abstract_inverted_index.line | 1054 |
| abstract_inverted_index.mo12 | 760 |
| abstract_inverted_index.more | 643 |
| abstract_inverted_index.most | 9 |
| abstract_inverted_index.node | 434 |
| abstract_inverted_index.poor | 44, 105 |
| abstract_inverted_index.rate | 562, 1071 |
| abstract_inverted_index.skin | 378 |
| abstract_inverted_index.than | 758, 771, 798, 818 |
| abstract_inverted_index.that | 1048, 1093 |
| abstract_inverted_index.this | 347, 703 |
| abstract_inverted_index.time | 658 |
| abstract_inverted_index.were | 283, 290, 573, 642, 878, 891, 1052 |
| abstract_inverted_index.with | 99, 203, 272, 275, 286, 340, 418, 502, 628, 639, 674, 688, 694, 893, 955, 1055, 1062, 1086 |
| abstract_inverted_index.(AEs) | 870 |
| abstract_inverted_index.(BCC) | 6 |
| abstract_inverted_index.(pts) | 202, 954 |
| abstract_inverted_index.20.0% | 615, 1014 |
| abstract_inverted_index.2016; | 138, 930 |
| abstract_inverted_index.2018; | 77 |
| abstract_inverted_index.2019; | 173 |
| abstract_inverted_index.2020; | 221, 973 |
| abstract_inverted_index.23.8% | 611 |
| abstract_inverted_index.30.0% | 568, 1010 |
| abstract_inverted_index.30.8% | 651 |
| abstract_inverted_index.33.3% | 997 |
| abstract_inverted_index.38.1% | 993 |
| abstract_inverted_index.50.0% | 564 |
| abstract_inverted_index.52.4% | 576 |
| abstract_inverted_index.69)71 | 375 |
| abstract_inverted_index.72)38 | 724 |
| abstract_inverted_index.72.7% | 671 |
| abstract_inverted_index.75)63 | 373 |
| abstract_inverted_index.Astra | 1127 |
| abstract_inverted_index.Basal | 3 |
| abstract_inverted_index.Chang | 57 |
| abstract_inverted_index.Debio | 1142 |
| abstract_inverted_index.Dirix | 59 |
| abstract_inverted_index.Given | 1092 |
| abstract_inverted_index.Grade | 983 |
| abstract_inverted_index.HPIs. | 1082 |
| abstract_inverted_index.ICIs. | 609, 894, 1068 |
| abstract_inverted_index.Lymph | 433, 454 |
| abstract_inverted_index.Merck | 1139 |
| abstract_inverted_index.Mohs6 | 429 |
| abstract_inverted_index.None6 | 396 |
| abstract_inverted_index.Peris | 189, 941 |
| abstract_inverted_index.Prior | 305 |
| abstract_inverted_index.Solid | 546 |
| abstract_inverted_index.These | 841 |
| abstract_inverted_index.Three | 488 |
| abstract_inverted_index.Treat | 75 |
| abstract_inverted_index.aBCC, | 252, 512 |
| abstract_inverted_index.after | 253, 329, 621 |
| abstract_inverted_index.basal | 19, 72, 125, 163, 206, 342, 465, 469, 514, 518, 690, 917, 958 |
| abstract_inverted_index.board | 256 |
| abstract_inverted_index.carry | 42 |
| abstract_inverted_index.clear | 244 |
| abstract_inverted_index.deep, | 30 |
| abstract_inverted_index.first | 1088 |
| abstract_inverted_index.found | 1047 |
| abstract_inverted_index.loss, | 883 |
| abstract_inverted_index.nevus | 288 |
| abstract_inverted_index.node1 | 455 |
| abstract_inverted_index.phase | 195, 947 |
| abstract_inverted_index.prior | 415, 499, 633 |
| abstract_inverted_index.range | 663 |
| abstract_inverted_index.rare, | 1096 |
| abstract_inverted_index.rates | 101, 1051 |
| abstract_inverted_index.size, | 1040 |
| abstract_inverted_index.small | 1038 |
| abstract_inverted_index.study | 1028 |
| abstract_inverted_index.table | 507, 855 |
| abstract_inverted_index.those | 590 |
| abstract_inverted_index.tumor | 630 |
| abstract_inverted_index.would | 1100 |
| abstract_inverted_index.year. | 623 |
| abstract_inverted_index.(0.0%) | 383, 387, 391, 395, 411, 424, 428, 432, 453, 458, 775, 816 |
| abstract_inverted_index.(20.0% | 565 |
| abstract_inverted_index.(47.6% | 581 |
| abstract_inverted_index.(61.1% | 649 |
| abstract_inverted_index.(ICIs) | 150 |
| abstract_inverted_index.(IQR); | 352, 361, 367, 485, 708, 714, 838 |
| abstract_inverted_index.(Table | 303, 587 |
| abstract_inverted_index.(aBCC) | 22 |
| abstract_inverted_index.A.L.S. | 58, 154 |
| abstract_inverted_index.Cannon | 157 |
| abstract_inverted_index.Common | 874 |
| abstract_inverted_index.Google | 88, 144, 184, 229, 936, 981 |
| abstract_inverted_index.Hughes | 110, 902 |
| abstract_inverted_index.J.G.D. | 158 |
| abstract_inverted_index.Median | 484, 837 |
| abstract_inverted_index.Merck, | 1138 |
| abstract_inverted_index.Oncol. | 220, 972 |
| abstract_inverted_index.Pharm. | 1143 |
| abstract_inverted_index.PubMed | 85, 141, 181, 933 |
| abstract_inverted_index.Sanofi | 1116 |
| abstract_inverted_index.Scopus | 86, 142, 182, 934 |
| abstract_inverted_index.Tumors | 547 |
| abstract_inverted_index.Within | 555 |
| abstract_inverted_index.alone, | 594 |
| abstract_inverted_index.and/or | 281, 698 |
| abstract_inverted_index.boards | 1111 |
| abstract_inverted_index.cancer | 379 |
| abstract_inverted_index.cases) | 299 |
| abstract_inverted_index.cohort | 580 |
| abstract_inverted_index.common | 10, 892 |
| abstract_inverted_index.events | 869 |
| abstract_inverted_index.immune | 147, 477, 526, 699, 834, 865 |
| abstract_inverted_index.large, | 29 |
| abstract_inverted_index.likely | 644 |
| abstract_inverted_index.muscle | 879, 1003 |
| abstract_inverted_index.pooled | 132, 924 |
| abstract_inverted_index.recent | 655 |
| abstract_inverted_index.record | 269 |
| abstract_inverted_index.repeat | 403, 491 |
| abstract_inverted_index.review | 130, 255, 262, 922 |
| abstract_inverted_index.sample | 1039 |
| abstract_inverted_index.served | 1122, 1147 |
| abstract_inverted_index.serves | 1107 |
| abstract_inverted_index.single | 1036 |
| abstract_inverted_index.subset | 37 |
| abstract_inverted_index.trends | 66 |
| abstract_inverted_index.values | 745, 785, 842 |
| abstract_inverted_index.varies | 237 |
| abstract_inverted_index.weight | 882 |
| abstract_inverted_index.(%).Age | 369 |
| abstract_inverted_index.(%).HPI | 354 |
| abstract_inverted_index.(%).† | 487, 840 |
| abstract_inverted_index.(0.0%)0 | 390, 394, 423, 431 |
| abstract_inverted_index.(10.0%) | 769 |
| abstract_inverted_index.(20.0%) | 802, 822 |
| abstract_inverted_index.(30.0%) | 739, 779, 809, 826 |
| abstract_inverted_index.(33.3%) | 598 |
| abstract_inverted_index.(4.8%)0 | 774, 828 |
| abstract_inverted_index.(4.8%)3 | 738, 778, 825 |
| abstract_inverted_index.(60.0%) | 762 |
| abstract_inverted_index.(66.7%) | 606 |
| abstract_inverted_index.(7.7%)0 | 389, 393, 422 |
| abstract_inverted_index.(7.7%)3 | 456 |
| abstract_inverted_index.(8.7%), | 324 |
| abstract_inverted_index.(9.5%)0 | 781 |
| abstract_inverted_index.(9.5%)2 | 821 |
| abstract_inverted_index.(HPIs), | 98 |
| abstract_inverted_index.(laBCC) | 209, 961 |
| abstract_inverted_index.(median | 657 |
| abstract_inverted_index.Adverse | 868 |
| abstract_inverted_index.Aldahan | 108, 900 |
| abstract_inverted_index.Editor: | 2 |
| abstract_inverted_index.Greater | 770, 817 |
| abstract_inverted_index.Locally | 440 |
| abstract_inverted_index.Nektar, | 1136 |
| abstract_inverted_index.Overall | 730 |
| abstract_inverted_index.Sanofi, | 1152 |
| abstract_inverted_index.Scholar | 89, 145, 230, 982 |
| abstract_inverted_index.Sekulic | 187, 939 |
| abstract_inverted_index.Surgery | 13 |
| abstract_inverted_index.Zeneca, | 1128 |
| abstract_inverted_index.absence | 241 |
| abstract_inverted_index.adrenal | 1023 |
| abstract_inverted_index.because | 238 |
| abstract_inverted_index.between | 1060 |
| abstract_inverted_index.cohort, | 558 |
| abstract_inverted_index.cramps, | 880, 1004 |
| abstract_inverted_index.despite | 647 |
| abstract_inverted_index.disease | 41, 449, 641 |
| abstract_inverted_index.further | 1102 |
| abstract_inverted_index.history | 337 |
| abstract_inverted_index.include | 746, 786, 843, 1029 |
| abstract_inverted_index.initial | 747, 787, 844 |
| abstract_inverted_index.locally | 26, 39, 121, 204, 297, 652, 679, 913, 956 |
| abstract_inverted_index.medical | 235, 268, 684 |
| abstract_inverted_index.months, | 662 |
| abstract_inverted_index.nature, | 1032 |
| abstract_inverted_index.overall | 1049 |
| abstract_inverted_index.partial | 550, 566, 582 |
| abstract_inverted_index.pathway | 96, 117, 474, 523, 696, 831, 862, 909 |
| abstract_inverted_index.patient | 413, 497 |
| abstract_inverted_index.reports | 1057 |
| abstract_inverted_index.results | 197, 949 |
| abstract_inverted_index.similar | 1059 |
| abstract_inverted_index.study.J | 169 |
| abstract_inverted_index.subtype | 439 |
| abstract_inverted_index.surgery | 52, 404, 492 |
| abstract_inverted_index.therapy | 91, 119, 312, 635, 685, 911 |
| abstract_inverted_index.treated | 274, 345, 693, 1085 |
| abstract_inverted_index.(%).ICI, | 363, 710 |
| abstract_inverted_index.(%).Time | 716 |
| abstract_inverted_index.(100.0%) | 444 |
| abstract_inverted_index.(12.5%)2 | 443, 462 |
| abstract_inverted_index.(14.3%)0 | 815 |
| abstract_inverted_index.(15.4%)2 | 385, 436 |
| abstract_inverted_index.(15.4%)3 | 409, 426 |
| abstract_inverted_index.(23.1%)2 | 381 |
| abstract_inverted_index.(23.1%)4 | 451 |
| abstract_inverted_index.(23.8%)1 | 768 |
| abstract_inverted_index.(25.0%)0 | 382, 386, 437 |
| abstract_inverted_index.(30.8%)7 | 446 |
| abstract_inverted_index.(33.3%)2 | 801 |
| abstract_inverted_index.(33.3%)3 | 808 |
| abstract_inverted_index.(37.5%)0 | 410, 427, 457 |
| abstract_inverted_index.(46.2%)0 | 430 |
| abstract_inverted_index.(46.2%)4 | 397 |
| abstract_inverted_index.(47.6%)2 | 735 |
| abstract_inverted_index.(47.6%)5 | 741 |
| abstract_inverted_index.(50.0%)0 | 452 |
| abstract_inverted_index.(50.0%)2 | 398 |
| abstract_inverted_index.(52.4%)5 | 732 |
| abstract_inverted_index.(56.5%), | 320 |
| abstract_inverted_index.(57.1%)6 | 761 |
| abstract_inverted_index.(69.2%)1 | 442, 461 |
| abstract_inverted_index.(87.5%)0 | 447 |
| abstract_inverted_index.74)Prior | 377 |
| abstract_inverted_index.Advanced | 18, 464, 513 |
| abstract_inverted_index.Arvinas, | 1135 |
| abstract_inverted_index.Burtness | 1121 |
| abstract_inverted_index.Celgene, | 1137 |
| abstract_inverted_index.Criteria | 544 |
| abstract_inverted_index.Distant3 | 450 |
| abstract_inverted_index.Emerging | 65 |
| abstract_inverted_index.Fatigue, | 886 |
| abstract_inverted_index.Hedgehog | 116, 830, 861, 908 |
| abstract_inverted_index.Hospital | 266 |
| abstract_inverted_index.Patients | 271, 285, 638 |
| abstract_inverted_index.Response | 542, 624 |
| abstract_inverted_index.Systemic | 90 |
| abstract_inverted_index.Thirteen | 317 |
| abstract_inverted_index.Unknown1 | 827 |
| abstract_inverted_index.Unknown2 | 780 |
| abstract_inverted_index.achieved | 618 |
| abstract_inverted_index.advanced | 27, 40, 71, 122, 162, 205, 298, 341, 680, 689, 914, 957 |
| abstract_inverted_index.advisory | 1110 |
| abstract_inverted_index.amenable | 50 |
| abstract_inverted_index.analysis | 133, 193, 925, 945 |
| abstract_inverted_index.appeared | 1077 |
| abstract_inverted_index.approval | 257 |
| abstract_inverted_index.baseline | 629 |
| abstract_inverted_index.category | 729 |
| abstract_inverted_index.clinical | 333, 1103 |
| abstract_inverted_index.colitis, | 1020 |
| abstract_inverted_index.compared | 1080 |
| abstract_inverted_index.complete | 553, 569, 585 |
| abstract_inverted_index.criteria | 302 |
| abstract_inverted_index.disease. | 33, 407 |
| abstract_inverted_index.followed | 355 |
| abstract_inverted_index.hedgehog | 95, 217, 473, 522, 695, 969 |
| abstract_inverted_index.impacted | 872 |
| abstract_inverted_index.includes | 94 |
| abstract_inverted_index.latency. | 1044 |
| abstract_inverted_index.months), | 667 |
| abstract_inverted_index.national | 1098 |
| abstract_inverted_index.observed | 574 |
| abstract_inverted_index.patients | 201, 293, 339, 401, 489, 673, 687, 752, 792, 849, 953, 999, 1016, 1061, 1084 |
| abstract_inverted_index.previous | 897, 1056 |
| abstract_inverted_index.progress | 211, 646, 963 |
| abstract_inverted_index.received | 310, 318, 322, 327, 1065, 1089 |
| abstract_inverted_index.registry | 1099 |
| abstract_inverted_index.response | 100, 152, 534, 551, 561, 620, 748, 788, 845, 1050 |
| abstract_inverted_index.resulted | 988 |
| abstract_inverted_index.slightly | 1078 |
| abstract_inverted_index.squamous | 481, 530 |
| abstract_inverted_index.surgery. | 316 |
| abstract_inverted_index.survival | 669 |
| abstract_inverted_index.syndrome | 289 |
| abstract_inverted_index.systemic | 307, 592 |
| abstract_inverted_index.therapy. | 873 |
| abstract_inverted_index.treating | 251 |
| abstract_inverted_index.uncommon | 233 |
| abstract_inverted_index.variable | 1042 |
| abstract_inverted_index.(fatigue, | 1002, 1019 |
| abstract_inverted_index.(months)6 | 722 |
| abstract_inverted_index.Dermatol. | 137, 172, 929 |
| abstract_inverted_index.Excision2 | 425 |
| abstract_inverted_index.Leventhal | 1106 |
| abstract_inverted_index.Melanoma2 | 384 |
| abstract_inverted_index.Multiple1 | 392 |
| abstract_inverted_index.Objective | 533, 571 |
| abstract_inverted_index.Oncology, | 1134 |
| abstract_inverted_index.Regeneron | 1118 |
| abstract_inverted_index.Similarly | 895 |
| abstract_inverted_index.Stratigos | 61 |
| abstract_inverted_index.Vaccinex, | 1132 |
| abstract_inverted_index.according | 540 |
| abstract_inverted_index.advanced9 | 441 |
| abstract_inverted_index.alopecia. | 885 |
| abstract_inverted_index.carcinoma | 5, 21, 208, 344, 692, 960 |
| abstract_inverted_index.criteria, | 548 |
| abstract_inverted_index.diagnosis | 660, 718 |
| abstract_inverted_index.diarrhea, | 887 |
| abstract_inverted_index.excluded. | 291 |
| abstract_inverted_index.favorable | 1079 |
| abstract_inverted_index.follow-up | 656 |
| abstract_inverted_index.generally | 48 |
| abstract_inverted_index.globally. | 12 |
| abstract_inverted_index.included. | 284 |
| abstract_inverted_index.including | 549 |
| abstract_inverted_index.inclusion | 301 |
| abstract_inverted_index.inhibitor | 118, 910 |
| abstract_inverted_index.objective | 560 |
| abstract_inverted_index.oncologic | 336, 538 |
| abstract_inverted_index.performed | 259 |
| abstract_inverted_index.prognosis | 45 |
| abstract_inverted_index.radiation | 54, 311, 634 |
| abstract_inverted_index.recurrent | 406, 494 |
| abstract_inverted_index.responded | 599, 607 |
| abstract_inverted_index.response) | 586 |
| abstract_inverted_index.response, | 567, 583 |
| abstract_inverted_index.response. | 554 |
| abstract_inverted_index.response1 | 737, 777, 824 |
| abstract_inverted_index.responses | 572 |
| abstract_inverted_index.secondary | 1072 |
| abstract_inverted_index.sustained | 619 |
| abstract_inverted_index.treatment | 69, 417, 501, 593, 648, 720, 728, 750, 790, 847, 990, 1043 |
| abstract_inverted_index.(0.0%)HPI, | 829 |
| abstract_inverted_index.(0.0%)aBCC | 438 |
| abstract_inverted_index.(HHIs).Ann | 219, 971 |
| abstract_inverted_index.(including | 28 |
| abstract_inverted_index.2∗Median | 366 |
| abstract_inverted_index.8∗Median | 360 |
| abstract_inverted_index.BioPharma, | 1130 |
| abstract_inverted_index.Evaluation | 543 |
| abstract_inverted_index.IIResponse | 682 |
| abstract_inverted_index.Metastatic | 34 |
| abstract_inverted_index.Radiation2 | 408 |
| abstract_inverted_index.Regeneron, | 1153 |
| abstract_inverted_index.advanced). | 653 |
| abstract_inverted_index.al.Primary | 192, 944 |
| abstract_inverted_index.anorexia), | 1006 |
| abstract_inverted_index.associated | 627 |
| abstract_inverted_index.carcinoma. | 532 |
| abstract_inverted_index.carcinoma: | 127, 165, 919 |
| abstract_inverted_index.carcinoma; | 467, 471, 516, 520 |
| abstract_inverted_index.cemiplimab | 199, 951 |
| abstract_inverted_index.checkpoint | 148, 478, 527, 700, 835, 866 |
| abstract_inverted_index.cohortHPI, | 348, 704 |
| abstract_inverted_index.consensus. | 245 |
| abstract_inverted_index.consultant | 1125, 1150 |
| abstract_inverted_index.determined | 536 |
| abstract_inverted_index.dysgeusia, | 881, 1005 |
| abstract_inverted_index.electronic | 267 |
| abstract_inverted_index.eventually | 754, 794, 851 |
| abstract_inverted_index.experience | 250 |
| abstract_inverted_index.facilitate | 1101 |
| abstract_inverted_index.first-line | 16 |
| abstract_inverted_index.frequently | 871 |
| abstract_inverted_index.inhibitor; | 475, 479, 524, 528 |
| abstract_inverted_index.inhibitors | 97, 149, 218, 697, 701, 970 |
| abstract_inverted_index.initiation | 721 |
| abstract_inverted_index.intolerant | 215, 967 |
| abstract_inverted_index.malignancy | 11 |
| abstract_inverted_index.management | 236 |
| abstract_inverted_index.metastatic | 24, 124, 640, 675, 916 |
| abstract_inverted_index.nivolumab, | 278 |
| abstract_inverted_index.response). | 570 |
| abstract_inverted_index.response10 | 734 |
| abstract_inverted_index.response11 | 731 |
| abstract_inverted_index.sonidegib, | 277 |
| abstract_inverted_index.systematic | 129, 921 |
| abstract_inverted_index.toxicities | 890 |
| abstract_inverted_index.treatment, | 308 |
| abstract_inverted_index.treatment. | 17 |
| abstract_inverted_index.(50.0%)Time | 742 |
| abstract_inverted_index.10∗Median | 713 |
| abstract_inverted_index.13∗Median | 351 |
| abstract_inverted_index.21∗Median | 707 |
| abstract_inverted_index.54)Response | 726 |
| abstract_inverted_index.Christensen | 1145 |
| abstract_inverted_index.Limitations | 1025 |
| abstract_inverted_index.Metastatic4 | 445 |
| abstract_inverted_index.Roche-Posay | 1114 |
| abstract_inverted_index.aggressive) | 32 |
| abstract_inverted_index.carboplatin | 419, 503 |
| abstract_inverted_index.cemiplimab, | 280 |
| abstract_inverted_index.diagnosis69 | 371 |
| abstract_inverted_index.disease.‡ | 495 |
| abstract_inverted_index.dissection2 | 435 |
| abstract_inverted_index.encompasses | 23 |
| abstract_inverted_index.inhibitors. | 867 |
| abstract_inverted_index.inhibitors; | 832, 863 |
| abstract_inverted_index.investigate | 247 |
| abstract_inverted_index.ipilimumab, | 279 |
| abstract_inverted_index.metastases9 | 460 |
| abstract_inverted_index.metastatic, | 295, 650 |
| abstract_inverted_index.paclitaxel. | 505 |
| abstract_inverted_index.vismodegib, | 276 |
| abstract_inverted_index.(2012-2022). | 270 |
| abstract_inverted_index.1-10Abstract | 79 |
| abstract_inverted_index.Macrogenics, | 1131 |
| abstract_inverted_index.Strasswimmer | 114, 906 |
| abstract_inverted_index.Twenty-three | 292 |
| abstract_inverted_index.chemotherapy | 416, 500 |
| abstract_inverted_index.dermatologic | 889 |
| abstract_inverted_index.institution, | 1037 |
| abstract_inverted_index.interruption | 991, 1008 |
| abstract_inverted_index.myocarditis, | 1021 |
| abstract_inverted_index.paclitaxel.1 | 421 |
| abstract_inverted_index.progression. | 756, 796, 853 |
| abstract_inverted_index.studies.JAMA | 136, 928 |
| abstract_inverted_index.thyroiditis, | 1022 |
| abstract_inverted_index.(100.0%)Prior | 399 |
| abstract_inverted_index.(100.0%)aBCC, | 463 |
| abstract_inverted_index.(34.8%).Table | 331 |
| abstract_inverted_index.Nevertheless, | 1045 |
| abstract_inverted_index.Progression10 | 740 |
| abstract_inverted_index.carcinoma.∗ | 483 |
| abstract_inverted_index.disease.Table | 681 |
| abstract_inverted_index.documentation | 539 |
| abstract_inverted_index.institutional | 249, 254 |
| abstract_inverted_index.pembrolizumab | 282 |
| abstract_inverted_index.rates).3Chang | 153 |
| abstract_inverted_index.retrospective | 261, 1031 |
| abstract_inverted_index.(0.0%)Duration | 782 |
| abstract_inverted_index.(50.0%)Partial | 733 |
| abstract_inverted_index.IDemographics, | 332 |
| abstract_inverted_index.inhibitors.∗ | 836 |
| abstract_inverted_index.interventional | 135, 927 |
| abstract_inverted_index.investigation. | 1104 |
| abstract_inverted_index.(20.0%)Complete | 736 |
| abstract_inverted_index.564-566Abstract | 175 |
| abstract_inverted_index.816-824Crossref | 140, 932 |
| abstract_inverted_index.discontinuation | 995, 1012, 1070 |
| abstract_inverted_index.insufficiency). | 1024 |
| abstract_inverted_index.therapy.1Migden | 55 |
| abstract_inverted_index.therapy†Three | 400 |
| abstract_inverted_index.(0.0%)Metastatic | 448 |
| abstract_inverted_index.Pharmaceuticals. | 1119 |
| abstract_inverted_index.al.Pembrolizumab | 160 |
| abstract_inverted_index.carcinoma.Cancer | 74 |
| abstract_inverted_index.characteristics, | 334 |
| abstract_inverted_index.disease-specific | 668 |
| abstract_inverted_index.lymphadenectomy. | 637 |
| abstract_inverted_index.pharmaceuticals. | 1156 |
| abstract_inverted_index.proof-of-concept | 168 |
| abstract_inverted_index.response†These | 744, 784 |
| abstract_inverted_index.reports,2Jacobsen | 898 |
| abstract_inverted_index.Chemotherapy‡One | 412 |
| abstract_inverted_index.Scholar,4Stratigos | 185, 937 |
| abstract_inverted_index.S1175-S1176Abstract | 223, 975 |
| abstract_inverted_index.tolerability,2Jacobsen | 106 |
| abstract_inverted_index.investigator-initiated, | 167 |
| cited_by_percentile_year.max | 95 |
| cited_by_percentile_year.min | 90 |
| corresponding_author_ids | https://openalex.org/A5041289456 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 10 |
| corresponding_institution_ids | https://openalex.org/I32971472 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.5699999928474426 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.63271215 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |