FDA Approval Summary: Nogapendekin Alfa Inbakicept-pmln with BCG for BCG-Unresponsive Carcinoma In Situ Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1158/1078-0432.ccr-25-1231
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1158/1078-0432.ccr-25-1231
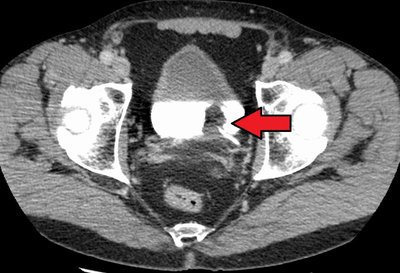
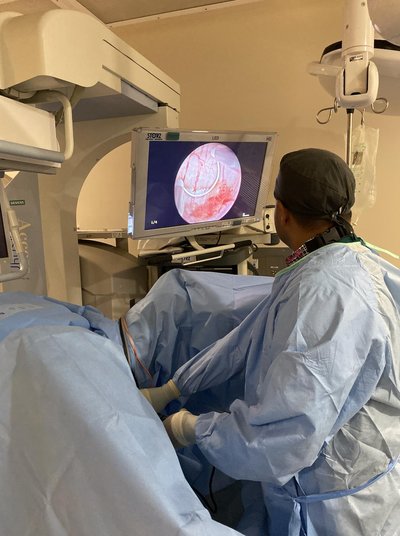
On April 22, 2024, the U.S. FDA granted regular approval to nogapendekin alfa inbakicept-pmln (N-803) with Bacillus Calmette-Guerin (BCG) for the treatment of adult patients with BCG-unresponsive non–muscle-invasive bladder cancer with carcinoma in situ, with or without papillary tumors. Substantial evidence of effectiveness for this application was obtained from cohort A of the single-arm, multicenter QUILT-3.032 trial. Patients received N-803 400 μg administered intravesically with TICE BCG once a week for 6 weeks as induction therapy, a second induction course if complete response (CR) was not achieved at month 3, and maintenance N-803 with BCG weekly for 3 weeks at months 4, 7, 10, 13, and 19 (for a total of 15 maintenance doses). The major efficacy outcome measures were CR at any time [as defined by negative results for cystoscopy [with transurethral resection of bladder tumor (TURBT)/biopsies as applicable] per local investigator assessment and urine cytology] and duration of response. The CR rate in the 77-patient efficacy population, per FDA review, was 62% [95% confidence interval (CI), 51%–73%]. Of the 48 patients with a CR, 28 (58%; 95% CI, 26%–55%) and 19 (40%; 95% CI, 16%–36%) maintained a response for ≥12 months and ≥24 months, respectively. The most common adverse reactions were increased creatinine, dysuria, hematuria, urinary frequency, urinary urgency, and urinary tract infection. This article summarizes the data and FDA thought process supporting the approval of N-803 with BCG.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1158/1078-0432.ccr-25-1231
- OA Status
- green
- References
- 11
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4413279695
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4413279695Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1158/1078-0432.ccr-25-1231Digital Object Identifier
- Title
-
FDA Approval Summary: Nogapendekin Alfa Inbakicept-pmln with BCG for BCG-Unresponsive Carcinoma In SituWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-08-18Full publication date if available
- Authors
-
Brian L. Heiss, Elaine Chang, Hee‐Koung Joeng, Mallorie H. Fiero, Lingshan Wang, Salaheldin S. Hamed, Haw-Jyh Chiu, Tiffany K. Ricks, Eun Ha Koh, Teegan A. Dellibovi-Ragheb, Min Wang, Christal Lee, William F. Pierce, John K. Leighton, Nam Atiqur Rahman, Shenghui Tang, Richard Pazdur, Laleh Amiri‐Kordestani, Paul G. Kluetz, Daniel L. SuzmanList of authors in order
- Landing page
-
https://doi.org/10.1158/1078-0432.ccr-25-1231Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
greenOpen access status per OpenAlex
- OA URL
-
https://www.ncbi.nlm.nih.gov/pmc/articles/12363661Direct OA link when available
- Concepts
-
Medicine, Cystoscopy, Carcinoma in situ, Bladder cancer, Population, Dysuria, Urinary system, Adverse effect, Surgery, Internal medicine, Urology, Cancer, Environmental healthTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- References (count)
-
11Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4413279695 |
|---|---|
| doi | https://doi.org/10.1158/1078-0432.ccr-25-1231 |
| ids.doi | https://doi.org/10.1158/1078-0432.ccr-25-1231 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/40824697 |
| ids.openalex | https://openalex.org/W4413279695 |
| fwci | 0.0 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D006801 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Humans |
| mesh[1].qualifier_ui | Q000188 |
| mesh[1].descriptor_ui | D001749 |
| mesh[1].is_major_topic | True |
| mesh[1].qualifier_name | drug therapy |
| mesh[1].descriptor_name | Urinary Bladder Neoplasms |
| mesh[2].qualifier_ui | Q000473 |
| mesh[2].descriptor_ui | D001749 |
| mesh[2].is_major_topic | True |
| mesh[2].qualifier_name | pathology |
| mesh[2].descriptor_name | Urinary Bladder Neoplasms |
| mesh[3].qualifier_ui | Q000008 |
| mesh[3].descriptor_ui | D001500 |
| mesh[3].is_major_topic | True |
| mesh[3].qualifier_name | administration & dosage |
| mesh[3].descriptor_name | BCG Vaccine |
| mesh[4].qualifier_ui | Q000009 |
| mesh[4].descriptor_ui | D001500 |
| mesh[4].is_major_topic | True |
| mesh[4].qualifier_name | adverse effects |
| mesh[4].descriptor_name | BCG Vaccine |
| mesh[5].qualifier_ui | |
| mesh[5].descriptor_ui | D014481 |
| mesh[5].is_major_topic | False |
| mesh[5].qualifier_name | |
| mesh[5].descriptor_name | United States |
| mesh[6].qualifier_ui | |
| mesh[6].descriptor_ui | D014486 |
| mesh[6].is_major_topic | False |
| mesh[6].qualifier_name | |
| mesh[6].descriptor_name | United States Food and Drug Administration |
| mesh[7].qualifier_ui | Q000188 |
| mesh[7].descriptor_ui | D002278 |
| mesh[7].is_major_topic | True |
| mesh[7].qualifier_name | drug therapy |
| mesh[7].descriptor_name | Carcinoma in Situ |
| mesh[8].qualifier_ui | Q000473 |
| mesh[8].descriptor_ui | D002278 |
| mesh[8].is_major_topic | True |
| mesh[8].qualifier_name | pathology |
| mesh[8].descriptor_name | Carcinoma in Situ |
| mesh[9].qualifier_ui | |
| mesh[9].descriptor_ui | D017277 |
| mesh[9].is_major_topic | False |
| mesh[9].qualifier_name | |
| mesh[9].descriptor_name | Drug Approval |
| mesh[10].qualifier_ui | |
| mesh[10].descriptor_ui | D005260 |
| mesh[10].is_major_topic | False |
| mesh[10].qualifier_name | |
| mesh[10].descriptor_name | Female |
| mesh[11].qualifier_ui | |
| mesh[11].descriptor_ui | D000368 |
| mesh[11].is_major_topic | False |
| mesh[11].qualifier_name | |
| mesh[11].descriptor_name | Aged |
| mesh[12].qualifier_ui | Q000627 |
| mesh[12].descriptor_ui | D000971 |
| mesh[12].is_major_topic | True |
| mesh[12].qualifier_name | therapeutic use |
| mesh[12].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[13].qualifier_ui | Q000009 |
| mesh[13].descriptor_ui | D000971 |
| mesh[13].is_major_topic | True |
| mesh[13].qualifier_name | adverse effects |
| mesh[13].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[14].qualifier_ui | |
| mesh[14].descriptor_ui | D008875 |
| mesh[14].is_major_topic | False |
| mesh[14].qualifier_name | |
| mesh[14].descriptor_name | Middle Aged |
| mesh[15].qualifier_ui | |
| mesh[15].descriptor_ui | D008297 |
| mesh[15].is_major_topic | False |
| mesh[15].qualifier_name | |
| mesh[15].descriptor_name | Male |
| mesh[16].qualifier_ui | Q000008 |
| mesh[16].descriptor_ui | D018796 |
| mesh[16].is_major_topic | True |
| mesh[16].qualifier_name | administration & dosage |
| mesh[16].descriptor_name | Immunoconjugates |
| mesh[17].qualifier_ui | Q000009 |
| mesh[17].descriptor_ui | D018796 |
| mesh[17].is_major_topic | True |
| mesh[17].qualifier_name | adverse effects |
| mesh[17].descriptor_name | Immunoconjugates |
| mesh[18].qualifier_ui | |
| mesh[18].descriptor_ui | D000328 |
| mesh[18].is_major_topic | False |
| mesh[18].qualifier_name | |
| mesh[18].descriptor_name | Adult |
| mesh[19].qualifier_ui | |
| mesh[19].descriptor_ui | D016896 |
| mesh[19].is_major_topic | False |
| mesh[19].qualifier_name | |
| mesh[19].descriptor_name | Treatment Outcome |
| mesh[20].qualifier_ui | |
| mesh[20].descriptor_ui | D006801 |
| mesh[20].is_major_topic | False |
| mesh[20].qualifier_name | |
| mesh[20].descriptor_name | Humans |
| mesh[21].qualifier_ui | Q000188 |
| mesh[21].descriptor_ui | D001749 |
| mesh[21].is_major_topic | True |
| mesh[21].qualifier_name | drug therapy |
| mesh[21].descriptor_name | Urinary Bladder Neoplasms |
| mesh[22].qualifier_ui | Q000473 |
| mesh[22].descriptor_ui | D001749 |
| mesh[22].is_major_topic | True |
| mesh[22].qualifier_name | pathology |
| mesh[22].descriptor_name | Urinary Bladder Neoplasms |
| mesh[23].qualifier_ui | Q000008 |
| mesh[23].descriptor_ui | D001500 |
| mesh[23].is_major_topic | True |
| mesh[23].qualifier_name | administration & dosage |
| mesh[23].descriptor_name | BCG Vaccine |
| mesh[24].qualifier_ui | Q000009 |
| mesh[24].descriptor_ui | D001500 |
| mesh[24].is_major_topic | True |
| mesh[24].qualifier_name | adverse effects |
| mesh[24].descriptor_name | BCG Vaccine |
| mesh[25].qualifier_ui | |
| mesh[25].descriptor_ui | D014481 |
| mesh[25].is_major_topic | False |
| mesh[25].qualifier_name | |
| mesh[25].descriptor_name | United States |
| mesh[26].qualifier_ui | |
| mesh[26].descriptor_ui | D014486 |
| mesh[26].is_major_topic | False |
| mesh[26].qualifier_name | |
| mesh[26].descriptor_name | United States Food and Drug Administration |
| mesh[27].qualifier_ui | Q000188 |
| mesh[27].descriptor_ui | D002278 |
| mesh[27].is_major_topic | True |
| mesh[27].qualifier_name | drug therapy |
| mesh[27].descriptor_name | Carcinoma in Situ |
| mesh[28].qualifier_ui | Q000473 |
| mesh[28].descriptor_ui | D002278 |
| mesh[28].is_major_topic | True |
| mesh[28].qualifier_name | pathology |
| mesh[28].descriptor_name | Carcinoma in Situ |
| mesh[29].qualifier_ui | |
| mesh[29].descriptor_ui | D017277 |
| mesh[29].is_major_topic | False |
| mesh[29].qualifier_name | |
| mesh[29].descriptor_name | Drug Approval |
| mesh[30].qualifier_ui | |
| mesh[30].descriptor_ui | D005260 |
| mesh[30].is_major_topic | False |
| mesh[30].qualifier_name | |
| mesh[30].descriptor_name | Female |
| mesh[31].qualifier_ui | |
| mesh[31].descriptor_ui | D000368 |
| mesh[31].is_major_topic | False |
| mesh[31].qualifier_name | |
| mesh[31].descriptor_name | Aged |
| mesh[32].qualifier_ui | Q000627 |
| mesh[32].descriptor_ui | D000971 |
| mesh[32].is_major_topic | True |
| mesh[32].qualifier_name | therapeutic use |
| mesh[32].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[33].qualifier_ui | Q000009 |
| mesh[33].descriptor_ui | D000971 |
| mesh[33].is_major_topic | True |
| mesh[33].qualifier_name | adverse effects |
| mesh[33].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[34].qualifier_ui | |
| mesh[34].descriptor_ui | D008875 |
| mesh[34].is_major_topic | False |
| mesh[34].qualifier_name | |
| mesh[34].descriptor_name | Middle Aged |
| mesh[35].qualifier_ui | |
| mesh[35].descriptor_ui | D008297 |
| mesh[35].is_major_topic | False |
| mesh[35].qualifier_name | |
| mesh[35].descriptor_name | Male |
| mesh[36].qualifier_ui | Q000008 |
| mesh[36].descriptor_ui | D018796 |
| mesh[36].is_major_topic | True |
| mesh[36].qualifier_name | administration & dosage |
| mesh[36].descriptor_name | Immunoconjugates |
| mesh[37].qualifier_ui | Q000009 |
| mesh[37].descriptor_ui | D018796 |
| mesh[37].is_major_topic | True |
| mesh[37].qualifier_name | adverse effects |
| mesh[37].descriptor_name | Immunoconjugates |
| mesh[38].qualifier_ui | |
| mesh[38].descriptor_ui | D000328 |
| mesh[38].is_major_topic | False |
| mesh[38].qualifier_name | |
| mesh[38].descriptor_name | Adult |
| mesh[39].qualifier_ui | |
| mesh[39].descriptor_ui | D016896 |
| mesh[39].is_major_topic | False |
| mesh[39].qualifier_name | |
| mesh[39].descriptor_name | Treatment Outcome |
| mesh[40].qualifier_ui | |
| mesh[40].descriptor_ui | D006801 |
| mesh[40].is_major_topic | False |
| mesh[40].qualifier_name | |
| mesh[40].descriptor_name | Humans |
| mesh[41].qualifier_ui | Q000188 |
| mesh[41].descriptor_ui | D001749 |
| mesh[41].is_major_topic | True |
| mesh[41].qualifier_name | drug therapy |
| mesh[41].descriptor_name | Urinary Bladder Neoplasms |
| mesh[42].qualifier_ui | Q000473 |
| mesh[42].descriptor_ui | D001749 |
| mesh[42].is_major_topic | True |
| mesh[42].qualifier_name | pathology |
| mesh[42].descriptor_name | Urinary Bladder Neoplasms |
| mesh[43].qualifier_ui | Q000008 |
| mesh[43].descriptor_ui | D001500 |
| mesh[43].is_major_topic | True |
| mesh[43].qualifier_name | administration & dosage |
| mesh[43].descriptor_name | BCG Vaccine |
| mesh[44].qualifier_ui | Q000009 |
| mesh[44].descriptor_ui | D001500 |
| mesh[44].is_major_topic | True |
| mesh[44].qualifier_name | adverse effects |
| mesh[44].descriptor_name | BCG Vaccine |
| mesh[45].qualifier_ui | |
| mesh[45].descriptor_ui | D014481 |
| mesh[45].is_major_topic | False |
| mesh[45].qualifier_name | |
| mesh[45].descriptor_name | United States |
| mesh[46].qualifier_ui | |
| mesh[46].descriptor_ui | D014486 |
| mesh[46].is_major_topic | False |
| mesh[46].qualifier_name | |
| mesh[46].descriptor_name | United States Food and Drug Administration |
| mesh[47].qualifier_ui | Q000188 |
| mesh[47].descriptor_ui | D002278 |
| mesh[47].is_major_topic | True |
| mesh[47].qualifier_name | drug therapy |
| mesh[47].descriptor_name | Carcinoma in Situ |
| mesh[48].qualifier_ui | Q000473 |
| mesh[48].descriptor_ui | D002278 |
| mesh[48].is_major_topic | True |
| mesh[48].qualifier_name | pathology |
| mesh[48].descriptor_name | Carcinoma in Situ |
| mesh[49].qualifier_ui | |
| mesh[49].descriptor_ui | D017277 |
| mesh[49].is_major_topic | False |
| mesh[49].qualifier_name | |
| mesh[49].descriptor_name | Drug Approval |
| type | article |
| title | FDA Approval Summary: Nogapendekin Alfa Inbakicept-pmln with BCG for BCG-Unresponsive Carcinoma In Situ |
| biblio.issue | 20 |
| biblio.volume | 31 |
| biblio.last_page | OF7 |
| biblio.first_page | OF1 |
| topics[0].id | https://openalex.org/T10458 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9987999796867371 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2746 |
| topics[0].subfield.display_name | Surgery |
| topics[0].display_name | Bladder and Urothelial Cancer Treatments |
| topics[1].id | https://openalex.org/T10158 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9952999949455261 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2730 |
| topics[1].subfield.display_name | Oncology |
| topics[1].display_name | Cancer Immunotherapy and Biomarkers |
| topics[2].id | https://openalex.org/T12992 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9929999709129333 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2740 |
| topics[2].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[2].display_name | Metastasis and carcinoma case studies |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.9101426601409912 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C2778769751 |
| concepts[1].level | 3 |
| concepts[1].score | 0.6265875101089478 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q246090 |
| concepts[1].display_name | Cystoscopy |
| concepts[2].id | https://openalex.org/C2777661416 |
| concepts[2].level | 3 |
| concepts[2].score | 0.5857376456260681 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q1035645 |
| concepts[2].display_name | Carcinoma in situ |
| concepts[3].id | https://openalex.org/C2780352672 |
| concepts[3].level | 3 |
| concepts[3].score | 0.5583509802818298 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q504775 |
| concepts[3].display_name | Bladder cancer |
| concepts[4].id | https://openalex.org/C2908647359 |
| concepts[4].level | 2 |
| concepts[4].score | 0.5090987086296082 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q2625603 |
| concepts[4].display_name | Population |
| concepts[5].id | https://openalex.org/C2776845205 |
| concepts[5].level | 3 |
| concepts[5].score | 0.5056884288787842 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q1269399 |
| concepts[5].display_name | Dysuria |
| concepts[6].id | https://openalex.org/C77411442 |
| concepts[6].level | 2 |
| concepts[6].score | 0.4515220522880554 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q181100 |
| concepts[6].display_name | Urinary system |
| concepts[7].id | https://openalex.org/C197934379 |
| concepts[7].level | 2 |
| concepts[7].score | 0.44656991958618164 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[7].display_name | Adverse effect |
| concepts[8].id | https://openalex.org/C141071460 |
| concepts[8].level | 1 |
| concepts[8].score | 0.4378122389316559 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q40821 |
| concepts[8].display_name | Surgery |
| concepts[9].id | https://openalex.org/C126322002 |
| concepts[9].level | 1 |
| concepts[9].score | 0.4347155690193176 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[9].display_name | Internal medicine |
| concepts[10].id | https://openalex.org/C126894567 |
| concepts[10].level | 1 |
| concepts[10].score | 0.4164380729198456 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q105650 |
| concepts[10].display_name | Urology |
| concepts[11].id | https://openalex.org/C121608353 |
| concepts[11].level | 2 |
| concepts[11].score | 0.40350234508514404 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[11].display_name | Cancer |
| concepts[12].id | https://openalex.org/C99454951 |
| concepts[12].level | 1 |
| concepts[12].score | 0.0 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q932068 |
| concepts[12].display_name | Environmental health |
| keywords[0].id | https://openalex.org/keywords/medicine |
| keywords[0].score | 0.9101426601409912 |
| keywords[0].display_name | Medicine |
| keywords[1].id | https://openalex.org/keywords/cystoscopy |
| keywords[1].score | 0.6265875101089478 |
| keywords[1].display_name | Cystoscopy |
| keywords[2].id | https://openalex.org/keywords/carcinoma-in-situ |
| keywords[2].score | 0.5857376456260681 |
| keywords[2].display_name | Carcinoma in situ |
| keywords[3].id | https://openalex.org/keywords/bladder-cancer |
| keywords[3].score | 0.5583509802818298 |
| keywords[3].display_name | Bladder cancer |
| keywords[4].id | https://openalex.org/keywords/population |
| keywords[4].score | 0.5090987086296082 |
| keywords[4].display_name | Population |
| keywords[5].id | https://openalex.org/keywords/dysuria |
| keywords[5].score | 0.5056884288787842 |
| keywords[5].display_name | Dysuria |
| keywords[6].id | https://openalex.org/keywords/urinary-system |
| keywords[6].score | 0.4515220522880554 |
| keywords[6].display_name | Urinary system |
| keywords[7].id | https://openalex.org/keywords/adverse-effect |
| keywords[7].score | 0.44656991958618164 |
| keywords[7].display_name | Adverse effect |
| keywords[8].id | https://openalex.org/keywords/surgery |
| keywords[8].score | 0.4378122389316559 |
| keywords[8].display_name | Surgery |
| keywords[9].id | https://openalex.org/keywords/internal-medicine |
| keywords[9].score | 0.4347155690193176 |
| keywords[9].display_name | Internal medicine |
| keywords[10].id | https://openalex.org/keywords/urology |
| keywords[10].score | 0.4164380729198456 |
| keywords[10].display_name | Urology |
| keywords[11].id | https://openalex.org/keywords/cancer |
| keywords[11].score | 0.40350234508514404 |
| keywords[11].display_name | Cancer |
| language | en |
| locations[0].id | doi:10.1158/1078-0432.ccr-25-1231 |
| locations[0].is_oa | False |
| locations[0].source.id | https://openalex.org/S183149254 |
| locations[0].source.issn | 1078-0432, 1557-3265 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1078-0432 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Clinical Cancer Research |
| locations[0].source.host_organization | https://openalex.org/P4310320273 |
| locations[0].source.host_organization_name | American Association for Cancer Research |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320273 |
| locations[0].license | |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Clinical Cancer Research |
| locations[0].landing_page_url | https://doi.org/10.1158/1078-0432.ccr-25-1231 |
| locations[1].id | pmid:40824697 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Clinical cancer research : an official journal of the American Association for Cancer Research |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/40824697 |
| locations[2].id | pmh:oai:pubmedcentral.nih.gov:12363661 |
| locations[2].is_oa | True |
| locations[2].source.id | https://openalex.org/S2764455111 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | PubMed Central |
| locations[2].source.host_organization | https://openalex.org/I1299303238 |
| locations[2].source.host_organization_name | National Institutes of Health |
| locations[2].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | Text |
| locations[2].license_id | |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | Clin Cancer Res |
| locations[2].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/12363661 |
| indexed_in | crossref, pubmed |
| authorships[0].author.id | https://openalex.org/A5035939498 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-7858-841X |
| authorships[0].author.display_name | Brian L. Heiss |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[0].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[0].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[0].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[0].institutions[0].type | facility |
| authorships[0].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[0].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[0].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[0].institutions[1].type | government |
| authorships[0].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[0].institutions[1].country_code | US |
| authorships[0].institutions[1].display_name | United States Food and Drug Administration |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Brian L. Heiss |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[1].author.id | https://openalex.org/A5079785100 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Elaine Chang |
| authorships[1].countries | US |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[1].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[1].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[1].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[1].institutions[0].type | facility |
| authorships[1].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[1].institutions[0].country_code | US |
| authorships[1].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[1].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[1].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[1].institutions[1].type | government |
| authorships[1].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[1].institutions[1].country_code | US |
| authorships[1].institutions[1].display_name | United States Food and Drug Administration |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Elaine Chang |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[2].author.id | https://openalex.org/A5028746842 |
| authorships[2].author.orcid | |
| authorships[2].author.display_name | Hee‐Koung Joeng |
| authorships[2].countries | US |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[2].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[2].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[2].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[2].institutions[0].type | facility |
| authorships[2].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[2].institutions[0].country_code | US |
| authorships[2].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[2].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[2].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[2].institutions[1].type | government |
| authorships[2].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[2].institutions[1].country_code | US |
| authorships[2].institutions[1].display_name | United States Food and Drug Administration |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Hee-Koung Joeng |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[3].author.id | https://openalex.org/A5084233916 |
| authorships[3].author.orcid | https://orcid.org/0000-0001-5536-116X |
| authorships[3].author.display_name | Mallorie H. Fiero |
| authorships[3].countries | US |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[3].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[3].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[3].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[3].institutions[0].type | facility |
| authorships[3].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[3].institutions[0].country_code | US |
| authorships[3].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[3].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[3].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[3].institutions[1].type | government |
| authorships[3].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[3].institutions[1].country_code | US |
| authorships[3].institutions[1].display_name | United States Food and Drug Administration |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Mallorie H. Fiero |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[4].author.id | https://openalex.org/A5059639133 |
| authorships[4].author.orcid | https://orcid.org/0000-0002-0852-3485 |
| authorships[4].author.display_name | Lingshan Wang |
| authorships[4].countries | US |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[4].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[4].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[4].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[4].institutions[0].type | facility |
| authorships[4].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[4].institutions[0].country_code | US |
| authorships[4].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[4].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[4].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[4].institutions[1].type | government |
| authorships[4].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[4].institutions[1].country_code | US |
| authorships[4].institutions[1].display_name | United States Food and Drug Administration |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Lingshan Wang |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[5].author.id | https://openalex.org/A5085195472 |
| authorships[5].author.orcid | https://orcid.org/0009-0009-7675-3088 |
| authorships[5].author.display_name | Salaheldin S. Hamed |
| authorships[5].countries | JP, US |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I142395110 |
| authorships[5].affiliations[0].raw_affiliation_string | 2Astellas Pharma, Tokyo, Japan. |
| authorships[5].affiliations[1].institution_ids | https://openalex.org/I1320320070 |
| authorships[5].affiliations[1].raw_affiliation_string | United States Food and Drug Administration, Silver Spring, MD, United States |
| authorships[5].institutions[0].id | https://openalex.org/I142395110 |
| authorships[5].institutions[0].ror | https://ror.org/01cjash87 |
| authorships[5].institutions[0].type | company |
| authorships[5].institutions[0].lineage | https://openalex.org/I142395110 |
| authorships[5].institutions[0].country_code | JP |
| authorships[5].institutions[0].display_name | Astellas Pharma (Japan) |
| authorships[5].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[5].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[5].institutions[1].type | government |
| authorships[5].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[5].institutions[1].country_code | US |
| authorships[5].institutions[1].display_name | United States Food and Drug Administration |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Salaheldin S. Hamed |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | 2Astellas Pharma, Tokyo, Japan., United States Food and Drug Administration, Silver Spring, MD, United States |
| authorships[6].author.id | https://openalex.org/A5020171435 |
| authorships[6].author.orcid | |
| authorships[6].author.display_name | Haw-Jyh Chiu |
| authorships[6].countries | US |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[6].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[6].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[6].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[6].institutions[0].type | facility |
| authorships[6].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[6].institutions[0].country_code | US |
| authorships[6].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[6].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[6].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[6].institutions[1].type | government |
| authorships[6].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[6].institutions[1].country_code | US |
| authorships[6].institutions[1].display_name | United States Food and Drug Administration |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Haw-Jyh Chiu |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[7].author.id | https://openalex.org/A5077762257 |
| authorships[7].author.orcid | |
| authorships[7].author.display_name | Tiffany K. Ricks |
| authorships[7].countries | US |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[7].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[7].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[7].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[7].institutions[0].type | facility |
| authorships[7].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[7].institutions[0].country_code | US |
| authorships[7].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[7].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[7].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[7].institutions[1].type | government |
| authorships[7].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[7].institutions[1].country_code | US |
| authorships[7].institutions[1].display_name | United States Food and Drug Administration |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Tiffany K. Ricks |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[8].author.id | https://openalex.org/A5112170957 |
| authorships[8].author.orcid | |
| authorships[8].author.display_name | Eun Ha Koh |
| authorships[8].countries | US |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[8].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[8].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[8].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[8].institutions[0].type | facility |
| authorships[8].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[8].institutions[0].country_code | US |
| authorships[8].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[8].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[8].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[8].institutions[1].type | government |
| authorships[8].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[8].institutions[1].country_code | US |
| authorships[8].institutions[1].display_name | United States Food and Drug Administration |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Eun Hee Koh |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[9].author.id | https://openalex.org/A5119330765 |
| authorships[9].author.orcid | |
| authorships[9].author.display_name | Teegan A. Dellibovi-Ragheb |
| authorships[9].countries | US |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[9].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[9].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[9].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[9].institutions[0].type | facility |
| authorships[9].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[9].institutions[0].country_code | US |
| authorships[9].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[9].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[9].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[9].institutions[1].type | government |
| authorships[9].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[9].institutions[1].country_code | US |
| authorships[9].institutions[1].display_name | United States Food and Drug Administration |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Teegan A. Dellibovi-Ragheb |
| authorships[9].is_corresponding | False |
| authorships[9].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[10].author.id | https://openalex.org/A5038660681 |
| authorships[10].author.orcid | |
| authorships[10].author.display_name | Min Wang |
| authorships[10].countries | US |
| authorships[10].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[10].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[10].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[10].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[10].institutions[0].type | facility |
| authorships[10].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[10].institutions[0].country_code | US |
| authorships[10].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[10].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[10].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[10].institutions[1].type | government |
| authorships[10].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[10].institutions[1].country_code | US |
| authorships[10].institutions[1].display_name | United States Food and Drug Administration |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Min Wang |
| authorships[10].is_corresponding | False |
| authorships[10].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[11].author.id | https://openalex.org/A5104217823 |
| authorships[11].author.orcid | |
| authorships[11].author.display_name | Christal Lee |
| authorships[11].countries | US |
| authorships[11].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[11].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[11].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[11].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[11].institutions[0].type | facility |
| authorships[11].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[11].institutions[0].country_code | US |
| authorships[11].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[11].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[11].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[11].institutions[1].type | government |
| authorships[11].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[11].institutions[1].country_code | US |
| authorships[11].institutions[1].display_name | United States Food and Drug Administration |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Christal Lee |
| authorships[11].is_corresponding | False |
| authorships[11].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[12].author.id | https://openalex.org/A5050956552 |
| authorships[12].author.orcid | https://orcid.org/0000-0002-3411-7720 |
| authorships[12].author.display_name | William F. Pierce |
| authorships[12].countries | US |
| authorships[12].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[12].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[12].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[12].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[12].institutions[0].type | facility |
| authorships[12].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[12].institutions[0].country_code | US |
| authorships[12].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[12].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[12].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[12].institutions[1].type | government |
| authorships[12].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[12].institutions[1].country_code | US |
| authorships[12].institutions[1].display_name | United States Food and Drug Administration |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | William F. Pierce |
| authorships[12].is_corresponding | False |
| authorships[12].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[13].author.id | https://openalex.org/A5110648169 |
| authorships[13].author.orcid | |
| authorships[13].author.display_name | John K. Leighton |
| authorships[13].countries | US |
| authorships[13].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[13].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[13].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[13].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[13].institutions[0].type | facility |
| authorships[13].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[13].institutions[0].country_code | US |
| authorships[13].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[13].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[13].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[13].institutions[1].type | government |
| authorships[13].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[13].institutions[1].country_code | US |
| authorships[13].institutions[1].display_name | United States Food and Drug Administration |
| authorships[13].author_position | middle |
| authorships[13].raw_author_name | John K. Leighton |
| authorships[13].is_corresponding | False |
| authorships[13].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[14].author.id | https://openalex.org/A5009175693 |
| authorships[14].author.orcid | https://orcid.org/0009-0001-0002-7006 |
| authorships[14].author.display_name | Nam Atiqur Rahman |
| authorships[14].countries | US |
| authorships[14].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[14].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[14].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[14].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[14].institutions[0].type | facility |
| authorships[14].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[14].institutions[0].country_code | US |
| authorships[14].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[14].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[14].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[14].institutions[1].type | government |
| authorships[14].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[14].institutions[1].country_code | US |
| authorships[14].institutions[1].display_name | United States Food and Drug Administration |
| authorships[14].author_position | middle |
| authorships[14].raw_author_name | Nam Atiqur Rahman |
| authorships[14].is_corresponding | False |
| authorships[14].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[15].author.id | https://openalex.org/A5043067852 |
| authorships[15].author.orcid | https://orcid.org/0000-0002-0437-665X |
| authorships[15].author.display_name | Shenghui Tang |
| authorships[15].countries | US |
| authorships[15].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[15].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[15].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[15].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[15].institutions[0].type | facility |
| authorships[15].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[15].institutions[0].country_code | US |
| authorships[15].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[15].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[15].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[15].institutions[1].type | government |
| authorships[15].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[15].institutions[1].country_code | US |
| authorships[15].institutions[1].display_name | United States Food and Drug Administration |
| authorships[15].author_position | middle |
| authorships[15].raw_author_name | Shenghui Tang |
| authorships[15].is_corresponding | False |
| authorships[15].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[16].author.id | https://openalex.org/A5051520990 |
| authorships[16].author.orcid | https://orcid.org/0000-0002-4771-9923 |
| authorships[16].author.display_name | Richard Pazdur |
| authorships[16].countries | US |
| authorships[16].affiliations[0].institution_ids | https://openalex.org/I1320320070 |
| authorships[16].affiliations[0].raw_affiliation_string | 3Oncology Center of Excellence (OCE), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[16].institutions[0].id | https://openalex.org/I1320320070 |
| authorships[16].institutions[0].ror | https://ror.org/034xvzb47 |
| authorships[16].institutions[0].type | government |
| authorships[16].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[16].institutions[0].country_code | US |
| authorships[16].institutions[0].display_name | United States Food and Drug Administration |
| authorships[16].author_position | middle |
| authorships[16].raw_author_name | Richard Pazdur |
| authorships[16].is_corresponding | False |
| authorships[16].raw_affiliation_strings | 3Oncology Center of Excellence (OCE), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[17].author.id | https://openalex.org/A5062916090 |
| authorships[17].author.orcid | https://orcid.org/0000-0002-0056-5437 |
| authorships[17].author.display_name | Laleh Amiri‐Kordestani |
| authorships[17].countries | US |
| authorships[17].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[17].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[17].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[17].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[17].institutions[0].type | facility |
| authorships[17].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[17].institutions[0].country_code | US |
| authorships[17].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[17].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[17].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[17].institutions[1].type | government |
| authorships[17].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[17].institutions[1].country_code | US |
| authorships[17].institutions[1].display_name | United States Food and Drug Administration |
| authorships[17].author_position | middle |
| authorships[17].raw_author_name | Laleh Amiri-Kordestani |
| authorships[17].is_corresponding | False |
| authorships[17].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[18].author.id | https://openalex.org/A5056460836 |
| authorships[18].author.orcid | https://orcid.org/0000-0002-9796-4791 |
| authorships[18].author.display_name | Paul G. Kluetz |
| authorships[18].countries | US |
| authorships[18].affiliations[0].institution_ids | https://openalex.org/I1320320070 |
| authorships[18].affiliations[0].raw_affiliation_string | United States Food and Drug Administration, Silver Spring, MD, United States |
| authorships[18].affiliations[1].institution_ids | https://openalex.org/I2801226506 |
| authorships[18].affiliations[1].raw_affiliation_string | 4Paradigm Health, Columbus, Ohio. |
| authorships[18].institutions[0].id | https://openalex.org/I2801226506 |
| authorships[18].institutions[0].ror | https://ror.org/0436zyg08 |
| authorships[18].institutions[0].type | government |
| authorships[18].institutions[0].lineage | https://openalex.org/I2801226506 |
| authorships[18].institutions[0].country_code | US |
| authorships[18].institutions[0].display_name | Ohio Department of Health |
| authorships[18].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[18].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[18].institutions[1].type | government |
| authorships[18].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[18].institutions[1].country_code | US |
| authorships[18].institutions[1].display_name | United States Food and Drug Administration |
| authorships[18].author_position | middle |
| authorships[18].raw_author_name | Paul G. Kluetz |
| authorships[18].is_corresponding | False |
| authorships[18].raw_affiliation_strings | 4Paradigm Health, Columbus, Ohio., United States Food and Drug Administration, Silver Spring, MD, United States |
| authorships[19].author.id | https://openalex.org/A5040090615 |
| authorships[19].author.orcid | https://orcid.org/0000-0002-1702-6114 |
| authorships[19].author.display_name | Daniel L. Suzman |
| authorships[19].countries | US |
| authorships[19].affiliations[0].institution_ids | https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[19].affiliations[0].raw_affiliation_string | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| authorships[19].institutions[0].id | https://openalex.org/I1333606569 |
| authorships[19].institutions[0].ror | https://ror.org/00yf3tm42 |
| authorships[19].institutions[0].type | facility |
| authorships[19].institutions[0].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070, https://openalex.org/I1333606569 |
| authorships[19].institutions[0].country_code | US |
| authorships[19].institutions[0].display_name | Center for Drug Evaluation and Research |
| authorships[19].institutions[1].id | https://openalex.org/I1320320070 |
| authorships[19].institutions[1].ror | https://ror.org/034xvzb47 |
| authorships[19].institutions[1].type | government |
| authorships[19].institutions[1].lineage | https://openalex.org/I1299022934, https://openalex.org/I1320320070 |
| authorships[19].institutions[1].country_code | US |
| authorships[19].institutions[1].display_name | United States Food and Drug Administration |
| authorships[19].author_position | last |
| authorships[19].raw_author_name | Daniel L. Suzman |
| authorships[19].is_corresponding | False |
| authorships[19].raw_affiliation_strings | 1Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, Silver Spring, Maryland. |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://www.ncbi.nlm.nih.gov/pmc/articles/12363661 |
| open_access.oa_status | green |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-08-19T00:00:00 |
| display_name | FDA Approval Summary: Nogapendekin Alfa Inbakicept-pmln with BCG for BCG-Unresponsive Carcinoma In Situ |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10458 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9987999796867371 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2746 |
| primary_topic.subfield.display_name | Surgery |
| primary_topic.display_name | Bladder and Urothelial Cancer Treatments |
| related_works | https://openalex.org/W2154965166, https://openalex.org/W1575075603, https://openalex.org/W2178187504, https://openalex.org/W3037610183, https://openalex.org/W2037888279, https://openalex.org/W2029203446, https://openalex.org/W2892025615, https://openalex.org/W3170858564, https://openalex.org/W2051498708, https://openalex.org/W1975705637 |
| cited_by_count | 0 |
| locations_count | 3 |
| best_oa_location.id | pmh:oai:pubmedcentral.nih.gov:12363661 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S2764455111 |
| best_oa_location.source.issn | |
| best_oa_location.source.type | repository |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | |
| best_oa_location.source.is_core | False |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | PubMed Central |
| best_oa_location.source.host_organization | https://openalex.org/I1299303238 |
| best_oa_location.source.host_organization_name | National Institutes of Health |
| best_oa_location.source.host_organization_lineage | https://openalex.org/I1299303238 |
| best_oa_location.license | |
| best_oa_location.pdf_url | |
| best_oa_location.version | submittedVersion |
| best_oa_location.raw_type | Text |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | False |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | Clin Cancer Res |
| best_oa_location.landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/12363661 |
| primary_location.id | doi:10.1158/1078-0432.ccr-25-1231 |
| primary_location.is_oa | False |
| primary_location.source.id | https://openalex.org/S183149254 |
| primary_location.source.issn | 1078-0432, 1557-3265 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1078-0432 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Clinical Cancer Research |
| primary_location.source.host_organization | https://openalex.org/P4310320273 |
| primary_location.source.host_organization_name | American Association for Cancer Research |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320273 |
| primary_location.license | |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Clinical Cancer Research |
| primary_location.landing_page_url | https://doi.org/10.1158/1078-0432.ccr-25-1231 |
| publication_date | 2025-08-18 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W1677810254, https://openalex.org/W2116299167, https://openalex.org/W4405637638, https://openalex.org/W4406431707, https://openalex.org/W3197808792, https://openalex.org/W2038973683, https://openalex.org/W2115953354, https://openalex.org/W1936300843, https://openalex.org/W2103567080, https://openalex.org/W4242888341, https://openalex.org/W4308713832 |
| referenced_works_count | 11 |
| abstract_inverted_index.3 | 98 |
| abstract_inverted_index.6 | 72 |
| abstract_inverted_index.A | 51 |
| abstract_inverted_index.a | 69, 77, 109, 175, 189 |
| abstract_inverted_index.15 | 112 |
| abstract_inverted_index.19 | 107, 183 |
| abstract_inverted_index.28 | 177 |
| abstract_inverted_index.3, | 90 |
| abstract_inverted_index.4, | 102 |
| abstract_inverted_index.48 | 172 |
| abstract_inverted_index.7, | 103 |
| abstract_inverted_index.CR | 121, 153 |
| abstract_inverted_index.Of | 170 |
| abstract_inverted_index.On | 1 |
| abstract_inverted_index.as | 74, 139 |
| abstract_inverted_index.at | 88, 100, 122 |
| abstract_inverted_index.by | 127 |
| abstract_inverted_index.if | 81 |
| abstract_inverted_index.in | 33, 155 |
| abstract_inverted_index.of | 23, 42, 52, 111, 135, 150, 228 |
| abstract_inverted_index.or | 36 |
| abstract_inverted_index.to | 11 |
| abstract_inverted_index.10, | 104 |
| abstract_inverted_index.13, | 105 |
| abstract_inverted_index.22, | 3 |
| abstract_inverted_index.400 | 61 |
| abstract_inverted_index.62% | 164 |
| abstract_inverted_index.95% | 179, 185 |
| abstract_inverted_index.BCG | 67, 95 |
| abstract_inverted_index.CI, | 180, 186 |
| abstract_inverted_index.CR, | 176 |
| abstract_inverted_index.FDA | 7, 161, 222 |
| abstract_inverted_index.The | 115, 152, 198 |
| abstract_inverted_index.[as | 125 |
| abstract_inverted_index.and | 91, 106, 145, 148, 182, 194, 212, 221 |
| abstract_inverted_index.any | 123 |
| abstract_inverted_index.for | 20, 44, 71, 97, 130, 191 |
| abstract_inverted_index.not | 86 |
| abstract_inverted_index.per | 141, 160 |
| abstract_inverted_index.the | 5, 21, 53, 156, 171, 219, 226 |
| abstract_inverted_index.was | 47, 85, 163 |
| abstract_inverted_index.μg | 62 |
| abstract_inverted_index.(CR) | 84 |
| abstract_inverted_index.(for | 108 |
| abstract_inverted_index.BCG. | 231 |
| abstract_inverted_index.TICE | 66 |
| abstract_inverted_index.This | 216 |
| abstract_inverted_index.U.S. | 6 |
| abstract_inverted_index.[95% | 165 |
| abstract_inverted_index.alfa | 13 |
| abstract_inverted_index.data | 220 |
| abstract_inverted_index.from | 49 |
| abstract_inverted_index.most | 199 |
| abstract_inverted_index.once | 68 |
| abstract_inverted_index.rate | 154 |
| abstract_inverted_index.this | 45 |
| abstract_inverted_index.time | 124 |
| abstract_inverted_index.week | 70 |
| abstract_inverted_index.were | 120, 203 |
| abstract_inverted_index.with | 16, 26, 31, 35, 65, 94, 174, 230 |
| abstract_inverted_index.(40%; | 184 |
| abstract_inverted_index.(58%; | 178 |
| abstract_inverted_index.(BCG) | 19 |
| abstract_inverted_index.(CI), | 168 |
| abstract_inverted_index.2024, | 4 |
| abstract_inverted_index.April | 2 |
| abstract_inverted_index.N-803 | 60, 93, 229 |
| abstract_inverted_index.[with | 132 |
| abstract_inverted_index.adult | 24 |
| abstract_inverted_index.local | 142 |
| abstract_inverted_index.major | 116 |
| abstract_inverted_index.month | 89 |
| abstract_inverted_index.situ, | 34 |
| abstract_inverted_index.total | 110 |
| abstract_inverted_index.tract | 214 |
| abstract_inverted_index.tumor | 137 |
| abstract_inverted_index.urine | 146 |
| abstract_inverted_index.weeks | 73, 99 |
| abstract_inverted_index.≥12 | 192 |
| abstract_inverted_index.≥24 | 195 |
| abstract_inverted_index.cancer | 30 |
| abstract_inverted_index.cohort | 50 |
| abstract_inverted_index.common | 200 |
| abstract_inverted_index.course | 80 |
| abstract_inverted_index.months | 101, 193 |
| abstract_inverted_index.second | 78 |
| abstract_inverted_index.trial. | 57 |
| abstract_inverted_index.weekly | 96 |
| abstract_inverted_index.(N-803) | 15 |
| abstract_inverted_index.adverse | 201 |
| abstract_inverted_index.article | 217 |
| abstract_inverted_index.bladder | 29, 136 |
| abstract_inverted_index.defined | 126 |
| abstract_inverted_index.doses). | 114 |
| abstract_inverted_index.granted | 8 |
| abstract_inverted_index.months, | 196 |
| abstract_inverted_index.outcome | 118 |
| abstract_inverted_index.process | 224 |
| abstract_inverted_index.regular | 9 |
| abstract_inverted_index.results | 129 |
| abstract_inverted_index.review, | 162 |
| abstract_inverted_index.thought | 223 |
| abstract_inverted_index.tumors. | 39 |
| abstract_inverted_index.urinary | 208, 210, 213 |
| abstract_inverted_index.without | 37 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.Bacillus | 17 |
| abstract_inverted_index.Patients | 58 |
| abstract_inverted_index.achieved | 87 |
| abstract_inverted_index.approval | 10, 227 |
| abstract_inverted_index.complete | 82 |
| abstract_inverted_index.duration | 149 |
| abstract_inverted_index.dysuria, | 206 |
| abstract_inverted_index.efficacy | 117, 158 |
| abstract_inverted_index.evidence | 41 |
| abstract_inverted_index.interval | 167 |
| abstract_inverted_index.measures | 119 |
| abstract_inverted_index.negative | 128 |
| abstract_inverted_index.obtained | 48 |
| abstract_inverted_index.patients | 25, 173 |
| abstract_inverted_index.received | 59 |
| abstract_inverted_index.response | 83, 190 |
| abstract_inverted_index.therapy, | 76 |
| abstract_inverted_index.urgency, | 211 |
| abstract_inverted_index.carcinoma | 32 |
| abstract_inverted_index.cytology] | 147 |
| abstract_inverted_index.increased | 204 |
| abstract_inverted_index.induction | 75, 79 |
| abstract_inverted_index.papillary | 38 |
| abstract_inverted_index.reactions | 202 |
| abstract_inverted_index.resection | 134 |
| abstract_inverted_index.response. | 151 |
| abstract_inverted_index.treatment | 22 |
| abstract_inverted_index.16%–36%) | 187 |
| abstract_inverted_index.26%–55%) | 181 |
| abstract_inverted_index.77-patient | 157 |
| abstract_inverted_index.assessment | 144 |
| abstract_inverted_index.confidence | 166 |
| abstract_inverted_index.cystoscopy | 131 |
| abstract_inverted_index.frequency, | 209 |
| abstract_inverted_index.hematuria, | 207 |
| abstract_inverted_index.infection. | 215 |
| abstract_inverted_index.maintained | 188 |
| abstract_inverted_index.summarizes | 218 |
| abstract_inverted_index.supporting | 225 |
| abstract_inverted_index.51%–73%]. | 169 |
| abstract_inverted_index.QUILT-3.032 | 56 |
| abstract_inverted_index.Substantial | 40 |
| abstract_inverted_index.applicable] | 140 |
| abstract_inverted_index.application | 46 |
| abstract_inverted_index.creatinine, | 205 |
| abstract_inverted_index.maintenance | 92, 113 |
| abstract_inverted_index.multicenter | 55 |
| abstract_inverted_index.population, | 159 |
| abstract_inverted_index.single-arm, | 54 |
| abstract_inverted_index.administered | 63 |
| abstract_inverted_index.investigator | 143 |
| abstract_inverted_index.nogapendekin | 12 |
| abstract_inverted_index.effectiveness | 43 |
| abstract_inverted_index.respectively. | 197 |
| abstract_inverted_index.transurethral | 133 |
| abstract_inverted_index.intravesically | 64 |
| abstract_inverted_index.Calmette-Guerin | 18 |
| abstract_inverted_index.inbakicept-pmln | 14 |
| abstract_inverted_index.(TURBT)/biopsies | 138 |
| abstract_inverted_index.BCG-unresponsive | 27 |
| abstract_inverted_index.non–muscle-invasive | 28 |
| cited_by_percentile_year | |
| countries_distinct_count | 2 |
| institutions_distinct_count | 20 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.7300000190734863 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.52729271 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | True |