Effect of the Oxide–Carbon Heterointerface on the Activity of Co3O4/NRGO Nanocomposites toward ORR and OER Article Swipe
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1021/acs.jpcc.6b00313
· OA: W2332493545
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1021/acs.jpcc.6b00313
· OA: W2332493545
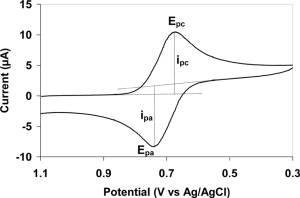
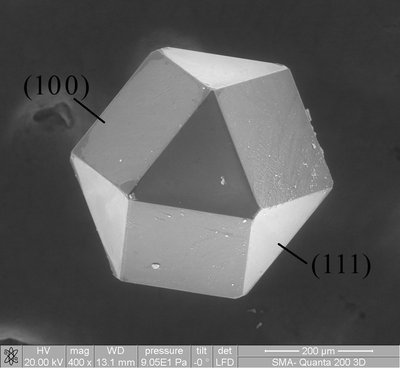
This work reports\nthe synthesis of Co<sub>3</sub>O<sub>4</sub> particles\nthat can be used as effective electrode materials for both the oxygen\nreduction (ORR) and evolution (OER) reactions. The development of\nsuch catalysts, free from precious group metals and capable of decreasing\noverpotentials in fuel cells, metal–air batteries, and water\nelectrolyzers, also requires stable supporting and conducting substrates\nin order to deposit low metal oxide loadings. This challenging approach\nled us to prepare Co<sub>3</sub>O<sub>4</sub> materials on graphene-based\ncomposites more stable than common used Vulcan carbon. Co<sub>3</sub>O<sub>4</sub> particles synthesized from a solvothermal method were\nthereby deposited onto reduced graphene oxide (RGO) and N-doped reduced\ngraphene oxide (NRGO) prepared from the Hummers method. The structural\nproperties and surface composition of the different materials characterized\nby X-ray diffraction, transmission electron microscopy, and X-ray\ninduced photoelectron spectroscopy measurements were combined to cyclic\nvoltammetry experiments for revealing the charge transfer from cobalt\nto nitrogen, which greatly affects the charge acceptance of the surface\nCo atoms. Electrochemical measurements provided sound evidence of\nactive and stable Co<sub>3</sub>O<sub>4</sub> catalysts toward the\nORR and OER.