Maturation of Na V and K V Channel Topographies in the Auditory Nerve Spike Initiator before and after Developmental Onset of Hearing Function Article Swipe
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1523/jneurosci.3437-15.2016
· OA: W2340813005
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1523/jneurosci.3437-15.2016
· OA: W2340813005
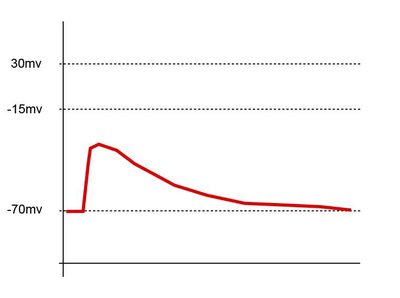
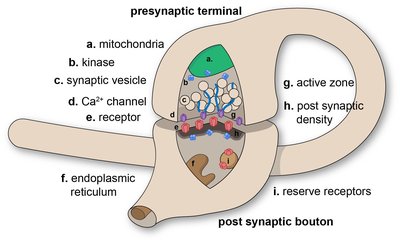
Auditory nerve excitation and thus hearing depend on spike-generating ion channels and their placement along the axons of auditory nerve fibers (ANFs). The developmental expression patterns and native axonal locations of voltage-gated ion channels in ANFs are unknown. Therefore, we examined the development of heminodes and nodes of Ranvier in the peripheral axons of type I ANFs in the rat cochlea with immunohistochemistry and confocal microscopy. Nodal structures presumably supporting presensory spiking formed between postnatal days 5 (P5) and P7, including Ankyrin-G, Na V 1.6, and Caspr. These immature nodal structures lacked low-voltage-activated K V 1.1 which was not enriched at juxtaparanodes until approximately P13, concurrent with the developmental onset of acoustic hearing function. Anatomical alignment of ANF spike-initiating heminodes relative to excitatory input from inner hair cell (IHC) ribbon synapses continued until approximately P30. High-voltage-activated K V 3.1b and K V 2.2 were expressed in mutually exclusive domains: K V 3.1b was strictly localized to nodes and heminodes, whereas K V 2.2 expression began at the juxtaparanodes and continued centrally along the first internode. At spike-initiating heminodes in the distal osseous spiral lamina, Na V 1.1 partly overlapped Na V 1.6 and ankyrin-G. ANFs displayed K V 7.2 and K V 7.3 at heminodes, nodes, internodes, and the unmyelinated synaptic terminal segments beneath IHCs in the organ of Corti. In response to sound, spikes are initiated at the heminode, which is tightly coupled to the IHC ribbon synapse ∼20–40 μm away. These results show that maturation of nodal alignment and ion channel content may underlie postnatal improvements of ANF excitability and discharge synchrony. SIGNIFICANCE STATEMENT Acoustic and electrical hearing depends on rapid, reliable, and precise spike generation in auditory nerve fibers. A limitation of current models and therapies is a lack of information on the identities and topographies of underlying ion channels. We report the developmental profile of the auditory nerve spike generator with a focus on Na V 1.1, Na V 1.6, K V 1.1, K V 2.2, K V 3.1b, K V 7.2, and K V 7.3 in relation to the scaffold ankyrin-G. Molecular anatomy of the spike generator matures in the weeks after developmental onset of hearing function. Subcellular positioning of voltage-gated ion channels will enable multicompartmental modeling of auditory nerve responses elicited by afferent chemical neurotransmission from hair cells and modulated by efferent neurotransmitters or evoked by extracellular field stimulation from a cochlear implant.