Structure of the voltage-gated K + channel Eag1 reveals an alternative voltage sensing mechanism Article Swipe
Related Concepts
Jonathan R. Whicher
,
Roderick MacKinnon
·
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1126/science.aaf8070
· OA: W2509636029
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1126/science.aaf8070
· OA: W2509636029
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1126/science.aaf8070
· OA: W2509636029
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1126/science.aaf8070
· OA: W2509636029
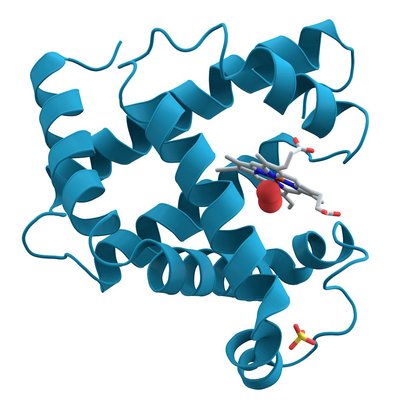
A different gate design The voltage-gated potassium channel Eag1 is overexpressed in tumor cells from a range of cancers, and inhibiting Eag1 reduces tumor growth. Whicher and Mackinnon determined the structure of a mammalian Eag1 bound to the inhibitor calmodulin at 3.78 Å resolution (see the Perspective by Toombes and Swartz). The organization of the voltage-sensing and pore domains differs from that of other potassium channels, indicating that the gating mechanism is distinct. The structure also shows how the channel can be closed by a ligand, independently of the position of the voltage sensor. Science , this issue p. 664 ; see also p. 646
Related Topics
Finding more related topics…