Evolutionary trend toward kinetic stability in the folding trajectory of RNases H Article Swipe
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1073/pnas.1611781113
· OA: W2545207659
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1073/pnas.1611781113
· OA: W2545207659
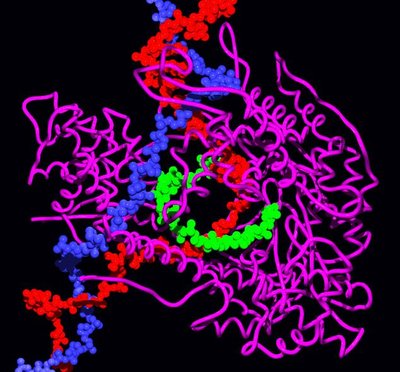
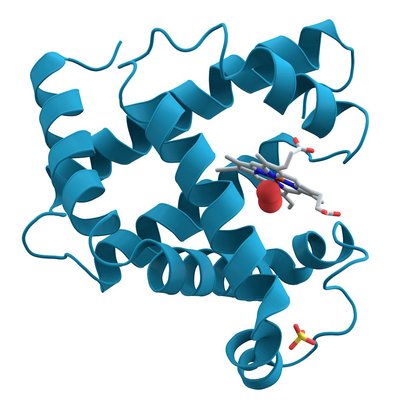
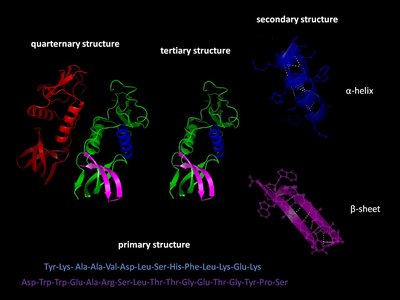
Significance Because protein folding is crucial to proper cellular function, there must be evolutionary pressures on how a protein achieves and maintains its folded structure. The outcome of these pressures on a folding pathway should be reflected in trends and patterns over a protein’s evolutionary history. To understand how folding pathways evolve, we characterized how reconstructed ancestral proteins of the ribonuclease H family fold. The deepest ancestors fold and unfold faster than their modern descendants, and kinetic stability evolved along both mesophilic and thermophilic lineages. This trend is possible because of a conserved partially folded intermediate state, which uncouples thermodynamic and kinetic stability to allow each parameter to evolve independently.