Oxidative‐Insertion Reactivity Across a Geometrically Constrained Metal→Borane Interaction Article Swipe
Related Concepts
Brandon R. Barnett
,
Michael L. Neville
,
Curtis E. Moore
,
Arnold L. Rheingold
,
Joshua S. Figueroa
·
 YOU?
·
· 2017
· Open Access
·
· DOI: https://doi.org/10.1002/anie.201702151
· OA: W2612462431
YOU?
·
· 2017
· Open Access
·
· DOI: https://doi.org/10.1002/anie.201702151
· OA: W2612462431
 YOU?
·
· 2017
· Open Access
·
· DOI: https://doi.org/10.1002/anie.201702151
· OA: W2612462431
YOU?
·
· 2017
· Open Access
·
· DOI: https://doi.org/10.1002/anie.201702151
· OA: W2612462431
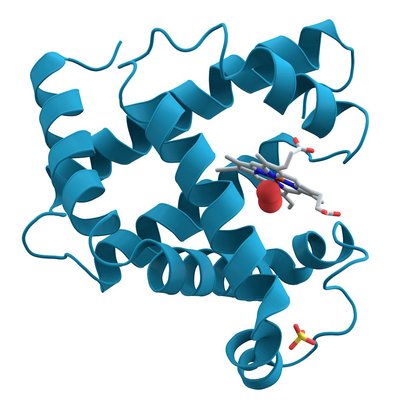
While interest in cooperative reactivity of transition metals and Lewis acids is receiving significant attention, the scope of known reactions that directly exploit the polarized reverse‐dative σ‐bond of metal‐borane complexes (i.e., M → BR 3 ) remains limited. Described herein is that the platinum (boryl)iminomethane (BIM) complex [Pt(κ 2 ‐N,B‐ Cy2 BIM)(CNAr Dipp2 )] can effect the oxidative insertion of a range of unsaturated organic substrates, including azides, isocyantes, and nitriles, as well as CO 2 and elemental sulfur (S 8 ). In addition, alkyl migration processes available to the BIM framework allow for post‐insertion reaction sequences resulting in product release from the metal center.
Related Topics
Finding more related topics…