Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime Article Swipe
Related Concepts
Microsecond
Photoexcitation
Copper
Luminescence
Excited state
Ligand (biochemistry)
Amide
Carbene
Photochemistry
Chemistry
Photoluminescence
Metal
Quantum efficiency
OLED
Materials science
Optoelectronics
Atomic physics
Physics
Optics
Layer (electronics)
Organic chemistry
Receptor
Catalysis
Biochemistry
Rasha Hamze
,
Jesse L. Peltier
,
Daniel Sylvinson
,
Moon Chul Jung
,
José Rodolfo Martínez y Cárdenas
,
Ralf Haiges
,
Michèle Soleilhavoup
,
Rodolphe Jazzar
,
Peter I. Djurovich
,
Guy Bertrand
,
Mark E. Thompson
·
 YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1126/science.aav2865
· OA: W2911951534
YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1126/science.aav2865
· OA: W2911951534
 YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1126/science.aav2865
· OA: W2911951534
YOU?
·
· 2019
· Open Access
·
· DOI: https://doi.org/10.1126/science.aav2865
· OA: W2911951534
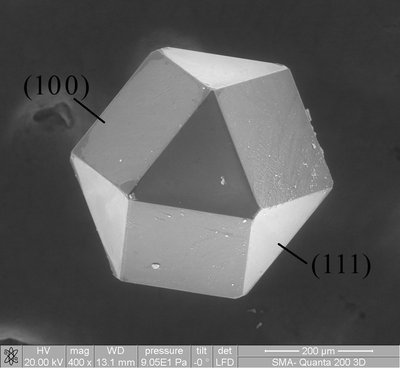
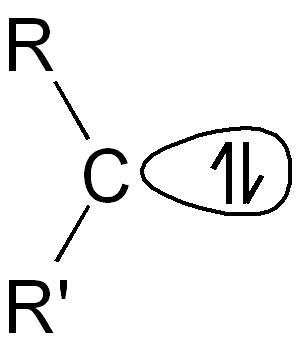
Helping copper glow Copper's abundance makes the metal an appealing candidate for luminescence applications. However, many copper complexes tend to decay nonradiatively after photoexcitation. A recently described exception involves a two-coordinate complex that sandwiches the metal between an amide ligand and a carbene ligand. Hamze et al. thoroughly explored this motif and measured a nearly perfect luminescence efficiency. They used this property to produce a prototype blue organic light-emitting diode. The photodynamics appeared largely ligand-centered, with the excited state attributed to copper-facilitated charge transfer from amide to carbene. Science , this issue p. 601
Related Topics
Finding more related topics…