Oxygen sensing ability of positronium atom for tumor hypoxia imaging Article Swipe
 YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.1038/s42005-020-00440-z
· OA: W3089479697
YOU?
·
· 2020
· Open Access
·
· DOI: https://doi.org/10.1038/s42005-020-00440-z
· OA: W3089479697
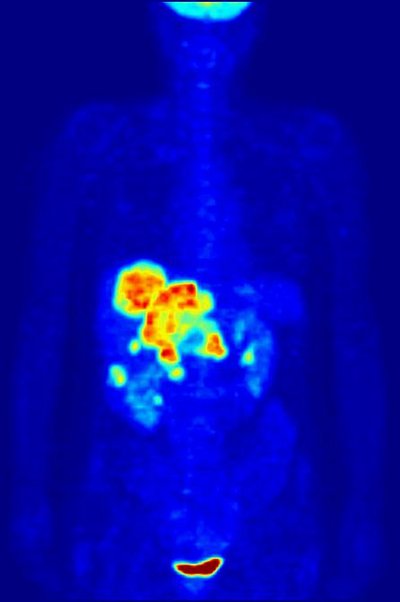
Positronium (Ps), a hydrogen-like atom consisting of a positron and an electron, is efficiently formed in the human body during positron emission tomography (PET) examination, and its decay rate into gamma-ray photons is significantly influenced by the chemical environment, especially the dissolved oxygen concentration (pO 2 ) due to the unpaired electrons. However, the functionality of PET has been underestimated by neglecting the specific information provided by Ps. By comparing the decay rates in O 2 -, N 2 -, and air-saturated waters, here we show that Ps probes the absolute value of pO 2 with a good linearity and a resolution better than 10 mmHg. This is a sufficient sensitivity for discriminating a hypoxic region in a tumor at approximately 6 mmHg from healthy tissues at approximately 40 mmHg. This method depends only on the fundamental properties of Ps and is independent of specific radiopharmaceuticals. The applications of Ps spin states and reactions will greatly enhance PET functionalities in the next decade.