Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease Article Swipe
 YOU?
·
· 2023
· Open Access
·
· DOI: https://doi.org/10.1001/jamaneurol.2023.1526
YOU?
·
· 2023
· Open Access
·
· DOI: https://doi.org/10.1001/jamaneurol.2023.1526
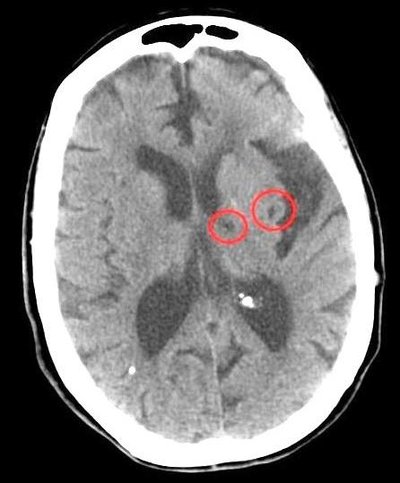
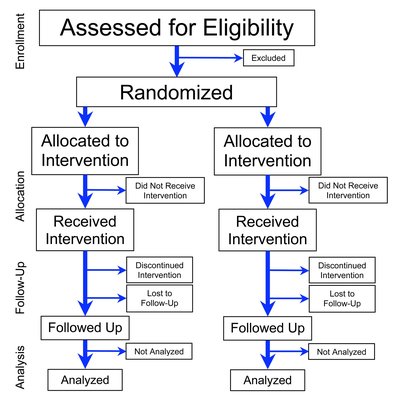
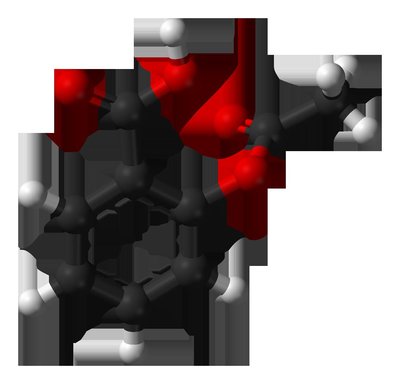
Importance Cerebral small vessel disease (cSVD) is a common cause of stroke (lacunar stroke), is the most common cause of vascular cognitive impairment, and impairs mobility and mood but has no specific treatment. Objective To test the feasibility, drug tolerability, safety, and effects of 1-year isosorbide mononitrate (ISMN) and cilostazol treatment on vascular, functional, and cognitive outcomes in patients with lacunar stroke. Design, Setting, and Participants The Lacunar Intervention Trial-2 (LACI-2) was an investigator-initiated, open-label, blinded end-point, randomized clinical trial with a 2 × 2 factorial design. The trial aimed to recruit 400 participants from 26 UK hospital stroke centers between February 5, 2018, and May 31, 2021, with 12-month follow-up. Included participants had clinical lacunar ischemic stroke, were independent, were aged older than 30 years, had compatible brain imaging findings, had capacity to consent, and had no contraindications to (or indications for) the study drugs. Data analysis was performed on August 12, 2022. Interventions All patients received guideline stroke prevention treatment and were randomized to ISMN (40-60 mg/d), cilostazol (200 mg/d), ISMN-cilostazol (40-60 and 200 mg/d, respectively), or no study drug. Main Outcomes The primary outcome was recruitment feasibility, including retention at 12 months. Secondary outcomes were safety (death), efficacy (composite of vascular events, dependence, cognition, and death), drug adherence, tolerability, recurrent stroke, dependence, cognitive impairment, quality of life (QOL), and hemorrhage. Results Of the 400 participants planned for this trial, 363 (90.8%) were recruited. Their median age was 64 (IQR, 56.0-72.0) years; 251 (69.1%) were men. The median time between stroke and randomization was 79 (IQR, 27.0-244.0) days. A total of 358 patients (98.6%) were retained in the study at 12 months, with 257 of 272 (94.5%) taking 50% or more of the allocated drug. Compared with those participants not receiving that particular drug, neither ISMN (adjusted hazard ratio [aHR], 0.80 [95% CI, 0.59 to 1.09]; P = .16) nor cilostazol (aHR, 0.77 [95% CI, 0.57 to 1.05]; P = .10) alone reduced the composite outcome in 297 patients. Isosorbide mononitrate reduced recurrent stroke in 353 patients (adjusted odds ratio [aOR], 0.23 [95% CI, 0.07 to 0.74]; P = .01) and cognitive impairment in 308 patients (aOR, 0.55 [95% CI, 0.36 to 0.86]; P = .008). Cilostazol reduced dependence in 320 patients (aHR, 0.31 [95% CI, 0.14 to 0.72]; P = .006). Combination ISMN-cilostazol reduced the composite (aHR, 0.58 [95% CI, 0.36 to 0.92]; P = .02), dependence (aOR, 0.14 [95% CI, 0.03 to 0.59]; P = .008), and any cognitive impairment (aOR, 0.44 [95% CI, 0.23 to 0.85]; P = .02) and improved QOL (adjusted mean difference, 0.10 [95% CI, 0.03 to 0.17]; P = .005) in 153 patients. There were no safety concerns. Conclusions and Relevance These results show that the LACI-2 trial was feasible and ISMN and cilostazol were well tolerated and safe. These agents may reduce recurrent stroke, dependence, and cognitive impairment after lacunar stroke, and they could prevent other adverse outcomes in cSVD. Therefore, both agents should be tested in large phase 3 trials. Trial Registration ClinicalTrials.gov Identifier: NCT03451591
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1001/jamaneurol.2023.1526
- https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdf
- OA Status
- hybrid
- Cited By
- 101
- References
- 30
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4377940039
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4377940039Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1001/jamaneurol.2023.1526Digital Object Identifier
- Title
-
Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel DiseaseWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2023Year of publication
- Publication date
-
2023-05-24Full publication date if available
- Authors
-
Joanna M. Wardlaw, Lisa J Woodhouse, Iris Mhlanga, Katherine Oatey, Anna K. Heye, John Bamford, Vera Cvoro, Fergus Doubal, Timothy J. England, Ahamad Hassan, Alan Montgomery, John T. O’Brien, Christine Roffe, Nikola Sprigg, David J. Werring, Philip M. Bath, Colin Baigent, Gary A. Ford, Jonathan Emberson, Alison D. Murray, A. Ross Naylor, Kailash Krishnan, Jesse Dawson, Chris Patterson, German Guzman Gutierrez, Stephen Makin, Usman Khan, L. Sztriha, Tom Booth, Amanathan Kirthivasan, Anwar Ijaz, Kirsty Harkness, Sevasti Ispoglou, Nigel Smyth, Aravinth Sivagnanaratnam, David Cohen, Lakshmanan Sekaran, Dinesh Chadha, Nasar Ahmad, Pratap Rana, Malik Azhar Hussain, Nic Weir, Thomas S. Harrison, Salim ElyasList of authors in order
- Landing page
-
https://doi.org/10.1001/jamaneurol.2023.1526Publisher landing page
- PDF URL
-
https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
hybridOpen access status per OpenAlex
- OA URL
-
https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdfDirect OA link when available
- Concepts
-
Cilostazol, Medicine, Tolerability, Lacunar stroke, Stroke (engine), Randomized controlled trial, Internal medicine, Isosorbide mononitrate, Physical therapy, Adverse effect, Aspirin, Ischemic stroke, Ischemia, Engineering, Mechanical engineeringTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
101Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 38, 2024: 46, 2023: 17Per-year citation counts (last 5 years)
- References (count)
-
30Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4377940039 |
|---|---|
| doi | https://doi.org/10.1001/jamaneurol.2023.1526 |
| ids.doi | https://doi.org/10.1001/jamaneurol.2023.1526 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/37222252 |
| ids.openalex | https://openalex.org/W4377940039 |
| fwci | 35.65849404 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D008297 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Male |
| mesh[1].qualifier_ui | |
| mesh[1].descriptor_ui | D006801 |
| mesh[1].is_major_topic | False |
| mesh[1].qualifier_name | |
| mesh[1].descriptor_name | Humans |
| mesh[2].qualifier_ui | |
| mesh[2].descriptor_ui | D000368 |
| mesh[2].is_major_topic | False |
| mesh[2].qualifier_name | |
| mesh[2].descriptor_name | Aged |
| mesh[3].qualifier_ui | |
| mesh[3].descriptor_ui | D008875 |
| mesh[3].is_major_topic | False |
| mesh[3].qualifier_name | |
| mesh[3].descriptor_name | Middle Aged |
| mesh[4].qualifier_ui | |
| mesh[4].descriptor_ui | D005260 |
| mesh[4].is_major_topic | False |
| mesh[4].qualifier_name | |
| mesh[4].descriptor_name | Female |
| mesh[5].qualifier_ui | Q000627 |
| mesh[5].descriptor_ui | D000077407 |
| mesh[5].is_major_topic | False |
| mesh[5].qualifier_name | therapeutic use |
| mesh[5].descriptor_name | Cilostazol |
| mesh[6].qualifier_ui | |
| mesh[6].descriptor_ui | D011788 |
| mesh[6].is_major_topic | False |
| mesh[6].qualifier_name | |
| mesh[6].descriptor_name | Quality of Life |
| mesh[7].qualifier_ui | Q000188 |
| mesh[7].descriptor_ui | D059409 |
| mesh[7].is_major_topic | True |
| mesh[7].qualifier_name | drug therapy |
| mesh[7].descriptor_name | Stroke, Lacunar |
| mesh[8].qualifier_ui | Q000209 |
| mesh[8].descriptor_ui | D020521 |
| mesh[8].is_major_topic | True |
| mesh[8].qualifier_name | etiology |
| mesh[8].descriptor_name | Stroke |
| mesh[9].qualifier_ui | Q000150 |
| mesh[9].descriptor_ui | D059345 |
| mesh[9].is_major_topic | True |
| mesh[9].qualifier_name | complications |
| mesh[9].descriptor_name | Cerebral Small Vessel Diseases |
| mesh[10].qualifier_ui | |
| mesh[10].descriptor_ui | D016896 |
| mesh[10].is_major_topic | False |
| mesh[10].qualifier_name | |
| mesh[10].descriptor_name | Treatment Outcome |
| mesh[11].qualifier_ui | |
| mesh[11].descriptor_ui | D008297 |
| mesh[11].is_major_topic | False |
| mesh[11].qualifier_name | |
| mesh[11].descriptor_name | Male |
| mesh[12].qualifier_ui | |
| mesh[12].descriptor_ui | D006801 |
| mesh[12].is_major_topic | False |
| mesh[12].qualifier_name | |
| mesh[12].descriptor_name | Humans |
| mesh[13].qualifier_ui | |
| mesh[13].descriptor_ui | D000368 |
| mesh[13].is_major_topic | False |
| mesh[13].qualifier_name | |
| mesh[13].descriptor_name | Aged |
| mesh[14].qualifier_ui | |
| mesh[14].descriptor_ui | D008875 |
| mesh[14].is_major_topic | False |
| mesh[14].qualifier_name | |
| mesh[14].descriptor_name | Middle Aged |
| mesh[15].qualifier_ui | |
| mesh[15].descriptor_ui | D005260 |
| mesh[15].is_major_topic | False |
| mesh[15].qualifier_name | |
| mesh[15].descriptor_name | Female |
| mesh[16].qualifier_ui | Q000627 |
| mesh[16].descriptor_ui | D000077407 |
| mesh[16].is_major_topic | False |
| mesh[16].qualifier_name | therapeutic use |
| mesh[16].descriptor_name | Cilostazol |
| mesh[17].qualifier_ui | |
| mesh[17].descriptor_ui | D011788 |
| mesh[17].is_major_topic | False |
| mesh[17].qualifier_name | |
| mesh[17].descriptor_name | Quality of Life |
| mesh[18].qualifier_ui | Q000188 |
| mesh[18].descriptor_ui | D059409 |
| mesh[18].is_major_topic | True |
| mesh[18].qualifier_name | drug therapy |
| mesh[18].descriptor_name | Stroke, Lacunar |
| mesh[19].qualifier_ui | Q000209 |
| mesh[19].descriptor_ui | D020521 |
| mesh[19].is_major_topic | True |
| mesh[19].qualifier_name | etiology |
| mesh[19].descriptor_name | Stroke |
| mesh[20].qualifier_ui | Q000150 |
| mesh[20].descriptor_ui | D059345 |
| mesh[20].is_major_topic | True |
| mesh[20].qualifier_name | complications |
| mesh[20].descriptor_name | Cerebral Small Vessel Diseases |
| mesh[21].qualifier_ui | |
| mesh[21].descriptor_ui | D016896 |
| mesh[21].is_major_topic | False |
| mesh[21].qualifier_name | |
| mesh[21].descriptor_name | Treatment Outcome |
| type | article |
| title | Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease |
| biblio.issue | 7 |
| biblio.volume | 80 |
| biblio.last_page | 682 |
| biblio.first_page | 682 |
| topics[0].id | https://openalex.org/T10227 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9993000030517578 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2713 |
| topics[0].subfield.display_name | Epidemiology |
| topics[0].display_name | Acute Ischemic Stroke Management |
| topics[1].id | https://openalex.org/T10816 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9918000102043152 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2740 |
| topics[1].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[1].display_name | Cerebrovascular and Carotid Artery Diseases |
| topics[2].id | https://openalex.org/T13796 |
| topics[2].field.id | https://openalex.org/fields/28 |
| topics[2].field.display_name | Neuroscience |
| topics[2].score | 0.9882000088691711 |
| topics[2].domain.id | https://openalex.org/domains/1 |
| topics[2].domain.display_name | Life Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2808 |
| topics[2].subfield.display_name | Neurology |
| topics[2].display_name | Neurological Disorders and Treatments |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C2779820409 |
| concepts[0].level | 3 |
| concepts[0].score | 0.8694508075714111 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q258591 |
| concepts[0].display_name | Cilostazol |
| concepts[1].id | https://openalex.org/C71924100 |
| concepts[1].level | 0 |
| concepts[1].score | 0.8287298083305359 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[1].display_name | Medicine |
| concepts[2].id | https://openalex.org/C2778375690 |
| concepts[2].level | 3 |
| concepts[2].score | 0.8076653480529785 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q7814214 |
| concepts[2].display_name | Tolerability |
| concepts[3].id | https://openalex.org/C2779285336 |
| concepts[3].level | 4 |
| concepts[3].score | 0.7211049795150757 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q6469117 |
| concepts[3].display_name | Lacunar stroke |
| concepts[4].id | https://openalex.org/C2780645631 |
| concepts[4].level | 2 |
| concepts[4].score | 0.5684263110160828 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q671554 |
| concepts[4].display_name | Stroke (engine) |
| concepts[5].id | https://openalex.org/C168563851 |
| concepts[5].level | 2 |
| concepts[5].score | 0.5129889845848083 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[5].display_name | Randomized controlled trial |
| concepts[6].id | https://openalex.org/C126322002 |
| concepts[6].level | 1 |
| concepts[6].score | 0.4904739558696747 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[6].display_name | Internal medicine |
| concepts[7].id | https://openalex.org/C2778492647 |
| concepts[7].level | 2 |
| concepts[7].score | 0.48889437317848206 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q423401 |
| concepts[7].display_name | Isosorbide mononitrate |
| concepts[8].id | https://openalex.org/C1862650 |
| concepts[8].level | 1 |
| concepts[8].score | 0.3943540155887604 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q186005 |
| concepts[8].display_name | Physical therapy |
| concepts[9].id | https://openalex.org/C197934379 |
| concepts[9].level | 2 |
| concepts[9].score | 0.2835471034049988 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[9].display_name | Adverse effect |
| concepts[10].id | https://openalex.org/C2777628954 |
| concepts[10].level | 2 |
| concepts[10].score | 0.14409193396568298 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q18216 |
| concepts[10].display_name | Aspirin |
| concepts[11].id | https://openalex.org/C3020199598 |
| concepts[11].level | 3 |
| concepts[11].score | 0.09821590781211853 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q12202 |
| concepts[11].display_name | Ischemic stroke |
| concepts[12].id | https://openalex.org/C541997718 |
| concepts[12].level | 2 |
| concepts[12].score | 0.0 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q188151 |
| concepts[12].display_name | Ischemia |
| concepts[13].id | https://openalex.org/C127413603 |
| concepts[13].level | 0 |
| concepts[13].score | 0.0 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q11023 |
| concepts[13].display_name | Engineering |
| concepts[14].id | https://openalex.org/C78519656 |
| concepts[14].level | 1 |
| concepts[14].score | 0.0 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q101333 |
| concepts[14].display_name | Mechanical engineering |
| keywords[0].id | https://openalex.org/keywords/cilostazol |
| keywords[0].score | 0.8694508075714111 |
| keywords[0].display_name | Cilostazol |
| keywords[1].id | https://openalex.org/keywords/medicine |
| keywords[1].score | 0.8287298083305359 |
| keywords[1].display_name | Medicine |
| keywords[2].id | https://openalex.org/keywords/tolerability |
| keywords[2].score | 0.8076653480529785 |
| keywords[2].display_name | Tolerability |
| keywords[3].id | https://openalex.org/keywords/lacunar-stroke |
| keywords[3].score | 0.7211049795150757 |
| keywords[3].display_name | Lacunar stroke |
| keywords[4].id | https://openalex.org/keywords/stroke |
| keywords[4].score | 0.5684263110160828 |
| keywords[4].display_name | Stroke (engine) |
| keywords[5].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[5].score | 0.5129889845848083 |
| keywords[5].display_name | Randomized controlled trial |
| keywords[6].id | https://openalex.org/keywords/internal-medicine |
| keywords[6].score | 0.4904739558696747 |
| keywords[6].display_name | Internal medicine |
| keywords[7].id | https://openalex.org/keywords/isosorbide-mononitrate |
| keywords[7].score | 0.48889437317848206 |
| keywords[7].display_name | Isosorbide mononitrate |
| keywords[8].id | https://openalex.org/keywords/physical-therapy |
| keywords[8].score | 0.3943540155887604 |
| keywords[8].display_name | Physical therapy |
| keywords[9].id | https://openalex.org/keywords/adverse-effect |
| keywords[9].score | 0.2835471034049988 |
| keywords[9].display_name | Adverse effect |
| keywords[10].id | https://openalex.org/keywords/aspirin |
| keywords[10].score | 0.14409193396568298 |
| keywords[10].display_name | Aspirin |
| keywords[11].id | https://openalex.org/keywords/ischemic-stroke |
| keywords[11].score | 0.09821590781211853 |
| keywords[11].display_name | Ischemic stroke |
| language | en |
| locations[0].id | doi:10.1001/jamaneurol.2023.1526 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S164389565 |
| locations[0].source.issn | 2168-6149, 2168-6157 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 2168-6149 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | JAMA Neurology |
| locations[0].source.host_organization | https://openalex.org/P4310320259 |
| locations[0].source.host_organization_name | American Medical Association |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320259 |
| locations[0].license | cc-by |
| locations[0].pdf_url | https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | JAMA Neurology |
| locations[0].landing_page_url | https://doi.org/10.1001/jamaneurol.2023.1526 |
| locations[1].id | pmid:37222252 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | JAMA neurology |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/37222252 |
| locations[2].id | pmh:oai:pubmedcentral.nih.gov:10209826 |
| locations[2].is_oa | True |
| locations[2].source.id | https://openalex.org/S2764455111 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | PubMed Central |
| locations[2].source.host_organization | https://openalex.org/I1299303238 |
| locations[2].source.host_organization_name | National Institutes of Health |
| locations[2].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[2].license | cc-by |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | Text |
| locations[2].license_id | https://openalex.org/licenses/cc-by |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | JAMA Neurol |
| locations[2].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/10209826 |
| locations[3].id | pmh:oai:pure.ed.ac.uk:publications/046b8213-1e23-406c-a796-0e70432652c2 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S4306400320 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | Edinburgh Research Explorer (University of Edinburgh) |
| locations[3].source.host_organization | https://openalex.org/I98677209 |
| locations[3].source.host_organization_name | University of Edinburgh |
| locations[3].source.host_organization_lineage | https://openalex.org/I98677209 |
| locations[3].license | cc-by |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | |
| locations[3].license_id | https://openalex.org/licenses/cc-by |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | |
| locations[3].landing_page_url | https://hdl.handle.net/20.500.11820/046b8213-1e23-406c-a796-0e70432652c2 |
| locations[4].id | pmh:oai:kclpure.kcl.ac.uk:publications/092a604f-acc5-4082-96d1-2424eec7ae1e |
| locations[4].is_oa | True |
| locations[4].source.id | https://openalex.org/S4306400063 |
| locations[4].source.issn | |
| locations[4].source.type | repository |
| locations[4].source.is_oa | False |
| locations[4].source.issn_l | |
| locations[4].source.is_core | False |
| locations[4].source.is_in_doaj | False |
| locations[4].source.display_name | Scopus (Elsevier) |
| locations[4].source.host_organization | |
| locations[4].source.host_organization_name | |
| locations[4].source.host_organization_lineage | |
| locations[4].license | other-oa |
| locations[4].pdf_url | |
| locations[4].version | submittedVersion |
| locations[4].raw_type | article |
| locations[4].license_id | https://openalex.org/licenses/other-oa |
| locations[4].is_accepted | False |
| locations[4].is_published | False |
| locations[4].raw_source_name | Wardlaw , J M , Woodhouse , L J , Mhlanga , I I , Oatey , K , Heye , A K , Bamford , J , Cvoro , V , Doubal , F N , England , T , Hassan , A , Montgomery , A , O’brien , J T , Roffe , C , Sprigg , N , Werring , D J , Bath , P M , Baigent , C , Ford , G , Emberson , J , Murray , A , Naylor , A R , Krishnan , K , Dawson , J , Patterson , C , Guzman gutierrez , G , Makin , S , Khan , U , Sztriha , L , Booth , T , Kirthivasan , A , Ijaz , A , Harkness , K , Ispoglou , S , Smyth , N , Sivagnanaratnam , A , Cohen , D , Sekaran , L , Chadha , D , Ahmad , N , Rana , P , Hussain , M , Weir , N , Harrison , T & Elyas , S 2023 , ' Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease : The Lacunar Intervention Trial-2 (LACI-2) Randomized Clinical Trial ' , JAMA Neurology , vol. 80 , no. 7 , pp. 682-692 . https://doi.org/10.1001/jamaneurol.2023.1526 |
| locations[4].landing_page_url | http://www.scopus.com/inward/record.url?scp=85164576886&partnerID=8YFLogxK |
| locations[5].id | pmh:oai:nottingham-repository.worktribe.com:21368790 |
| locations[5].is_oa | True |
| locations[5].source.id | https://openalex.org/S4306402481 |
| locations[5].source.issn | |
| locations[5].source.type | repository |
| locations[5].source.is_oa | False |
| locations[5].source.issn_l | |
| locations[5].source.is_core | False |
| locations[5].source.is_in_doaj | False |
| locations[5].source.display_name | Repository@Nottingham (University of Nottingham) |
| locations[5].source.host_organization | https://openalex.org/I142263535 |
| locations[5].source.host_organization_name | University of Nottingham |
| locations[5].source.host_organization_lineage | https://openalex.org/I142263535 |
| locations[5].license | cc-by |
| locations[5].pdf_url | |
| locations[5].version | submittedVersion |
| locations[5].raw_type | Journal Article |
| locations[5].license_id | https://openalex.org/licenses/cc-by |
| locations[5].is_accepted | False |
| locations[5].is_published | False |
| locations[5].raw_source_name | |
| locations[5].landing_page_url | https://nottingham-repository.worktribe.com/output/21368790 |
| indexed_in | crossref, pubmed |
| authorships[0].author.id | https://openalex.org/A5004889443 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-9812-6642 |
| authorships[0].author.display_name | Joanna M. Wardlaw |
| authorships[0].countries | GB |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I4210126463, https://openalex.org/I98677209 |
| authorships[0].affiliations[0].raw_affiliation_string | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[0].institutions[0].id | https://openalex.org/I4210126463 |
| authorships[0].institutions[0].ror | https://ror.org/02wedp412 |
| authorships[0].institutions[0].type | facility |
| authorships[0].institutions[0].lineage | https://openalex.org/I4210126463 |
| authorships[0].institutions[0].country_code | GB |
| authorships[0].institutions[0].display_name | UK Dementia Research Institute |
| authorships[0].institutions[1].id | https://openalex.org/I98677209 |
| authorships[0].institutions[1].ror | https://ror.org/01nrxwf90 |
| authorships[0].institutions[1].type | education |
| authorships[0].institutions[1].lineage | https://openalex.org/I98677209 |
| authorships[0].institutions[1].country_code | GB |
| authorships[0].institutions[1].display_name | University of Edinburgh |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Joanna M. Wardlaw |
| authorships[0].is_corresponding | True |
| authorships[0].raw_affiliation_strings | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[1].author.id | https://openalex.org/A5026156735 |
| authorships[1].author.orcid | https://orcid.org/0000-0002-4472-1999 |
| authorships[1].author.display_name | Lisa J Woodhouse |
| authorships[1].countries | GB |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[1].affiliations[0].raw_affiliation_string | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[1].institutions[0].id | https://openalex.org/I142263535 |
| authorships[1].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[1].institutions[0].country_code | GB |
| authorships[1].institutions[0].display_name | University of Nottingham |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Lisa J. Woodhouse |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[2].author.id | https://openalex.org/A5002579195 |
| authorships[2].author.orcid | https://orcid.org/0000-0003-3475-1630 |
| authorships[2].author.display_name | Iris Mhlanga |
| authorships[2].countries | GB |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[2].affiliations[0].raw_affiliation_string | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[2].institutions[0].id | https://openalex.org/I142263535 |
| authorships[2].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[2].institutions[0].type | education |
| authorships[2].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[2].institutions[0].country_code | GB |
| authorships[2].institutions[0].display_name | University of Nottingham |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Iris I. Mhlanga |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[3].author.id | https://openalex.org/A5018184790 |
| authorships[3].author.orcid | https://orcid.org/0000-0002-4667-9763 |
| authorships[3].author.display_name | Katherine Oatey |
| authorships[3].countries | GB |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I98677209 |
| authorships[3].affiliations[0].raw_affiliation_string | Edinburgh Clinical Trials Unit, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[3].institutions[0].id | https://openalex.org/I98677209 |
| authorships[3].institutions[0].ror | https://ror.org/01nrxwf90 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I98677209 |
| authorships[3].institutions[0].country_code | GB |
| authorships[3].institutions[0].display_name | University of Edinburgh |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Katherine Oatey |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Edinburgh Clinical Trials Unit, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[4].author.id | https://openalex.org/A5005462505 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Anna K. Heye |
| authorships[4].countries | GB |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I98677209 |
| authorships[4].affiliations[0].raw_affiliation_string | Edinburgh Clinical Trials Unit, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[4].institutions[0].id | https://openalex.org/I98677209 |
| authorships[4].institutions[0].ror | https://ror.org/01nrxwf90 |
| authorships[4].institutions[0].type | education |
| authorships[4].institutions[0].lineage | https://openalex.org/I98677209 |
| authorships[4].institutions[0].country_code | GB |
| authorships[4].institutions[0].display_name | University of Edinburgh |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Anna K. Heye |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Edinburgh Clinical Trials Unit, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[5].author.id | https://openalex.org/A5051317564 |
| authorships[5].author.orcid | https://orcid.org/0000-0002-7817-8832 |
| authorships[5].author.display_name | John Bamford |
| authorships[5].countries | GB |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I1295121038 |
| authorships[5].affiliations[0].raw_affiliation_string | Department of Neurology, Leeds General Infirmary, Leeds, United Kingdom |
| authorships[5].institutions[0].id | https://openalex.org/I1295121038 |
| authorships[5].institutions[0].ror | https://ror.org/04hrjej96 |
| authorships[5].institutions[0].type | healthcare |
| authorships[5].institutions[0].lineage | https://openalex.org/I1295121038, https://openalex.org/I2799390153 |
| authorships[5].institutions[0].country_code | GB |
| authorships[5].institutions[0].display_name | Leeds General Infirmary |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | John Bamford |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Department of Neurology, Leeds General Infirmary, Leeds, United Kingdom |
| authorships[6].author.id | https://openalex.org/A5020521035 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-3913-5100 |
| authorships[6].author.display_name | Vera Cvoro |
| authorships[6].countries | GB |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I4210126463, https://openalex.org/I98677209 |
| authorships[6].affiliations[0].raw_affiliation_string | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[6].affiliations[1].institution_ids | https://openalex.org/I125671874, https://openalex.org/I4210134181 |
| authorships[6].affiliations[1].raw_affiliation_string | Victoria Hospital, National Health Service Fife, Kirkcaldy, United Kingdom |
| authorships[6].institutions[0].id | https://openalex.org/I125671874 |
| authorships[6].institutions[0].ror | https://ror.org/02wnqcb97 |
| authorships[6].institutions[0].type | healthcare |
| authorships[6].institutions[0].lineage | https://openalex.org/I125671874, https://openalex.org/I1311074006 |
| authorships[6].institutions[0].country_code | GB |
| authorships[6].institutions[0].display_name | National Health Service |
| authorships[6].institutions[1].id | https://openalex.org/I4210126463 |
| authorships[6].institutions[1].ror | https://ror.org/02wedp412 |
| authorships[6].institutions[1].type | facility |
| authorships[6].institutions[1].lineage | https://openalex.org/I4210126463 |
| authorships[6].institutions[1].country_code | GB |
| authorships[6].institutions[1].display_name | UK Dementia Research Institute |
| authorships[6].institutions[2].id | https://openalex.org/I98677209 |
| authorships[6].institutions[2].ror | https://ror.org/01nrxwf90 |
| authorships[6].institutions[2].type | education |
| authorships[6].institutions[2].lineage | https://openalex.org/I98677209 |
| authorships[6].institutions[2].country_code | GB |
| authorships[6].institutions[2].display_name | University of Edinburgh |
| authorships[6].institutions[3].id | https://openalex.org/I4210134181 |
| authorships[6].institutions[3].ror | https://ror.org/02stzb903 |
| authorships[6].institutions[3].type | healthcare |
| authorships[6].institutions[3].lineage | https://openalex.org/I2803037235, https://openalex.org/I4210134181 |
| authorships[6].institutions[3].country_code | GB |
| authorships[6].institutions[3].display_name | Victoria Hospital |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Vera Cvoro |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom, Victoria Hospital, National Health Service Fife, Kirkcaldy, United Kingdom |
| authorships[7].author.id | https://openalex.org/A5015592473 |
| authorships[7].author.orcid | https://orcid.org/0000-0002-2769-3148 |
| authorships[7].author.display_name | Fergus Doubal |
| authorships[7].countries | GB |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I4210126463, https://openalex.org/I98677209 |
| authorships[7].affiliations[0].raw_affiliation_string | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[7].institutions[0].id | https://openalex.org/I4210126463 |
| authorships[7].institutions[0].ror | https://ror.org/02wedp412 |
| authorships[7].institutions[0].type | facility |
| authorships[7].institutions[0].lineage | https://openalex.org/I4210126463 |
| authorships[7].institutions[0].country_code | GB |
| authorships[7].institutions[0].display_name | UK Dementia Research Institute |
| authorships[7].institutions[1].id | https://openalex.org/I98677209 |
| authorships[7].institutions[1].ror | https://ror.org/01nrxwf90 |
| authorships[7].institutions[1].type | education |
| authorships[7].institutions[1].lineage | https://openalex.org/I98677209 |
| authorships[7].institutions[1].country_code | GB |
| authorships[7].institutions[1].display_name | University of Edinburgh |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Fergus N. Doubal |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom |
| authorships[8].author.id | https://openalex.org/A5013869274 |
| authorships[8].author.orcid | https://orcid.org/0000-0001-5330-8584 |
| authorships[8].author.display_name | Timothy J. England |
| authorships[8].countries | GB |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[8].affiliations[0].raw_affiliation_string | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[8].institutions[0].id | https://openalex.org/I142263535 |
| authorships[8].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[8].institutions[0].type | education |
| authorships[8].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[8].institutions[0].country_code | GB |
| authorships[8].institutions[0].display_name | University of Nottingham |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Timothy England |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[9].author.id | https://openalex.org/A5039240225 |
| authorships[9].author.orcid | https://orcid.org/0000-0001-9716-8587 |
| authorships[9].author.display_name | Ahamad Hassan |
| authorships[9].countries | GB |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I1295121038 |
| authorships[9].affiliations[0].raw_affiliation_string | Department of Neurology, Leeds General Infirmary, Leeds, United Kingdom |
| authorships[9].institutions[0].id | https://openalex.org/I1295121038 |
| authorships[9].institutions[0].ror | https://ror.org/04hrjej96 |
| authorships[9].institutions[0].type | healthcare |
| authorships[9].institutions[0].lineage | https://openalex.org/I1295121038, https://openalex.org/I2799390153 |
| authorships[9].institutions[0].country_code | GB |
| authorships[9].institutions[0].display_name | Leeds General Infirmary |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Ahamad Hassan |
| authorships[9].is_corresponding | False |
| authorships[9].raw_affiliation_strings | Department of Neurology, Leeds General Infirmary, Leeds, United Kingdom |
| authorships[10].author.id | https://openalex.org/A5046999262 |
| authorships[10].author.orcid | https://orcid.org/0000-0003-0450-1606 |
| authorships[10].author.display_name | Alan Montgomery |
| authorships[10].countries | GB |
| authorships[10].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[10].affiliations[0].raw_affiliation_string | Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, United Kingdom |
| authorships[10].institutions[0].id | https://openalex.org/I142263535 |
| authorships[10].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[10].institutions[0].type | education |
| authorships[10].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[10].institutions[0].country_code | GB |
| authorships[10].institutions[0].display_name | University of Nottingham |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Alan Montgomery |
| authorships[10].is_corresponding | False |
| authorships[10].raw_affiliation_strings | Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, United Kingdom |
| authorships[11].author.id | https://openalex.org/A5014262891 |
| authorships[11].author.orcid | https://orcid.org/0000-0002-0837-5080 |
| authorships[11].author.display_name | John T. O’Brien |
| authorships[11].countries | GB |
| authorships[11].affiliations[0].institution_ids | https://openalex.org/I241749 |
| authorships[11].affiliations[0].raw_affiliation_string | Department of Psychiatry, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom |
| authorships[11].institutions[0].id | https://openalex.org/I241749 |
| authorships[11].institutions[0].ror | https://ror.org/013meh722 |
| authorships[11].institutions[0].type | education |
| authorships[11].institutions[0].lineage | https://openalex.org/I241749 |
| authorships[11].institutions[0].country_code | GB |
| authorships[11].institutions[0].display_name | University of Cambridge |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | John T. O’Brien |
| authorships[11].is_corresponding | False |
| authorships[11].raw_affiliation_strings | Department of Psychiatry, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom |
| authorships[12].author.id | https://openalex.org/A5077260495 |
| authorships[12].author.orcid | https://orcid.org/0000-0002-5259-6649 |
| authorships[12].author.display_name | Christine Roffe |
| authorships[12].countries | GB |
| authorships[12].affiliations[0].institution_ids | https://openalex.org/I56007636 |
| authorships[12].affiliations[0].raw_affiliation_string | Stroke Research, Keele University, Stoke-on-Trent, United Kingdom |
| authorships[12].institutions[0].id | https://openalex.org/I56007636 |
| authorships[12].institutions[0].ror | https://ror.org/00340yn33 |
| authorships[12].institutions[0].type | education |
| authorships[12].institutions[0].lineage | https://openalex.org/I56007636 |
| authorships[12].institutions[0].country_code | GB |
| authorships[12].institutions[0].display_name | Keele University |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | Christine Roffe |
| authorships[12].is_corresponding | False |
| authorships[12].raw_affiliation_strings | Stroke Research, Keele University, Stoke-on-Trent, United Kingdom |
| authorships[13].author.id | https://openalex.org/A5057794391 |
| authorships[13].author.orcid | https://orcid.org/0000-0002-5871-8168 |
| authorships[13].author.display_name | Nikola Sprigg |
| authorships[13].countries | GB |
| authorships[13].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[13].affiliations[0].raw_affiliation_string | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[13].institutions[0].id | https://openalex.org/I142263535 |
| authorships[13].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[13].institutions[0].type | education |
| authorships[13].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[13].institutions[0].country_code | GB |
| authorships[13].institutions[0].display_name | University of Nottingham |
| authorships[13].author_position | middle |
| authorships[13].raw_author_name | Nikola Sprigg |
| authorships[13].is_corresponding | False |
| authorships[13].raw_affiliation_strings | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[14].author.id | https://openalex.org/A5088937468 |
| authorships[14].author.orcid | https://orcid.org/0000-0003-2074-1861 |
| authorships[14].author.display_name | David J. Werring |
| authorships[14].countries | GB |
| authorships[14].affiliations[0].institution_ids | https://openalex.org/I4210151618, https://openalex.org/I45129253 |
| authorships[14].affiliations[0].raw_affiliation_string | Stroke Research Centre, University College London Queen Square Institute of Neurology, Russell Square House, London, United Kingdom |
| authorships[14].institutions[0].id | https://openalex.org/I4210151618 |
| authorships[14].institutions[0].ror | https://ror.org/048b34d51 |
| authorships[14].institutions[0].type | healthcare |
| authorships[14].institutions[0].lineage | https://openalex.org/I1340918713, https://openalex.org/I4210151618 |
| authorships[14].institutions[0].country_code | GB |
| authorships[14].institutions[0].display_name | National Hospital for Neurology and Neurosurgery |
| authorships[14].institutions[1].id | https://openalex.org/I45129253 |
| authorships[14].institutions[1].ror | https://ror.org/02jx3x895 |
| authorships[14].institutions[1].type | education |
| authorships[14].institutions[1].lineage | https://openalex.org/I124357947, https://openalex.org/I45129253 |
| authorships[14].institutions[1].country_code | GB |
| authorships[14].institutions[1].display_name | University College London |
| authorships[14].author_position | middle |
| authorships[14].raw_author_name | David J. Werring |
| authorships[14].is_corresponding | False |
| authorships[14].raw_affiliation_strings | Stroke Research Centre, University College London Queen Square Institute of Neurology, Russell Square House, London, United Kingdom |
| authorships[15].author.id | https://openalex.org/A5078222721 |
| authorships[15].author.orcid | https://orcid.org/0000-0003-2734-5132 |
| authorships[15].author.display_name | Philip M. Bath |
| authorships[15].countries | GB |
| authorships[15].affiliations[0].institution_ids | https://openalex.org/I142263535 |
| authorships[15].affiliations[0].raw_affiliation_string | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[15].institutions[0].id | https://openalex.org/I142263535 |
| authorships[15].institutions[0].ror | https://ror.org/01ee9ar58 |
| authorships[15].institutions[0].type | education |
| authorships[15].institutions[0].lineage | https://openalex.org/I142263535 |
| authorships[15].institutions[0].country_code | GB |
| authorships[15].institutions[0].display_name | University of Nottingham |
| authorships[15].author_position | middle |
| authorships[15].raw_author_name | Philip M. Bath |
| authorships[15].is_corresponding | False |
| authorships[15].raw_affiliation_strings | Stroke Trials Unit, Mental Health and Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom |
| authorships[16].author.id | https://openalex.org/A5030243760 |
| authorships[16].author.orcid | https://orcid.org/0000-0003-4856-7420 |
| authorships[16].author.display_name | Colin Baigent |
| authorships[16].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[16].author_position | middle |
| authorships[16].raw_author_name | Colin Baigent |
| authorships[16].is_corresponding | False |
| authorships[16].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[17].author.id | https://openalex.org/A5064918196 |
| authorships[17].author.orcid | https://orcid.org/0000-0001-8719-4968 |
| authorships[17].author.display_name | Gary A. Ford |
| authorships[17].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[17].author_position | middle |
| authorships[17].raw_author_name | Gary Ford |
| authorships[17].is_corresponding | False |
| authorships[17].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[18].author.id | https://openalex.org/A5030250235 |
| authorships[18].author.orcid | https://orcid.org/0000-0001-7792-9422 |
| authorships[18].author.display_name | Jonathan Emberson |
| authorships[18].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[18].author_position | middle |
| authorships[18].raw_author_name | Jonathan Emberson |
| authorships[18].is_corresponding | False |
| authorships[18].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[19].author.id | https://openalex.org/A5011437964 |
| authorships[19].author.orcid | https://orcid.org/0000-0003-4915-4847 |
| authorships[19].author.display_name | Alison D. Murray |
| authorships[19].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[19].author_position | middle |
| authorships[19].raw_author_name | Alison Murray |
| authorships[19].is_corresponding | False |
| authorships[19].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[20].author.id | https://openalex.org/A5110258959 |
| authorships[20].author.orcid | |
| authorships[20].author.display_name | A. Ross Naylor |
| authorships[20].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[20].author_position | middle |
| authorships[20].raw_author_name | A Ross Naylor |
| authorships[20].is_corresponding | False |
| authorships[20].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[21].author.id | https://openalex.org/A5062746761 |
| authorships[21].author.orcid | https://orcid.org/0000-0002-6486-3783 |
| authorships[21].author.display_name | Kailash Krishnan |
| authorships[21].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[21].author_position | middle |
| authorships[21].raw_author_name | Kailash Krishnan |
| authorships[21].is_corresponding | False |
| authorships[21].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[22].author.id | https://openalex.org/A5066328545 |
| authorships[22].author.orcid | https://orcid.org/0000-0001-7532-2475 |
| authorships[22].author.display_name | Jesse Dawson |
| authorships[22].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[22].author_position | middle |
| authorships[22].raw_author_name | Jesse Dawson |
| authorships[22].is_corresponding | False |
| authorships[22].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[23].author.id | https://openalex.org/A5045333516 |
| authorships[23].author.orcid | https://orcid.org/0000-0002-5914-8718 |
| authorships[23].author.display_name | Chris Patterson |
| authorships[23].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[23].author_position | middle |
| authorships[23].raw_author_name | Chris Patterson |
| authorships[23].is_corresponding | False |
| authorships[23].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[24].author.id | https://openalex.org/A5078994193 |
| authorships[24].author.orcid | |
| authorships[24].author.display_name | German Guzman Gutierrez |
| authorships[24].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[24].author_position | middle |
| authorships[24].raw_author_name | German Guzman Gutierrez |
| authorships[24].is_corresponding | False |
| authorships[24].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[25].author.id | https://openalex.org/A5045827546 |
| authorships[25].author.orcid | https://orcid.org/0000-0001-8701-9043 |
| authorships[25].author.display_name | Stephen Makin |
| authorships[25].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[25].author_position | middle |
| authorships[25].raw_author_name | Stephen Makin |
| authorships[25].is_corresponding | False |
| authorships[25].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[26].author.id | https://openalex.org/A5036054782 |
| authorships[26].author.orcid | https://orcid.org/0000-0002-5692-3600 |
| authorships[26].author.display_name | Usman Khan |
| authorships[26].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[26].author_position | middle |
| authorships[26].raw_author_name | Usman Khan |
| authorships[26].is_corresponding | False |
| authorships[26].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[27].author.id | https://openalex.org/A5030327357 |
| authorships[27].author.orcid | https://orcid.org/0000-0003-4639-3180 |
| authorships[27].author.display_name | L. Sztriha |
| authorships[27].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[27].author_position | middle |
| authorships[27].raw_author_name | Laszlo Sztriha |
| authorships[27].is_corresponding | False |
| authorships[27].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[28].author.id | https://openalex.org/A5000473787 |
| authorships[28].author.orcid | https://orcid.org/0000-0002-4235-1381 |
| authorships[28].author.display_name | Tom Booth |
| authorships[28].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[28].author_position | middle |
| authorships[28].raw_author_name | Thomas Booth |
| authorships[28].is_corresponding | False |
| authorships[28].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[29].author.id | https://openalex.org/A5092006301 |
| authorships[29].author.orcid | |
| authorships[29].author.display_name | Amanathan Kirthivasan |
| authorships[29].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[29].author_position | middle |
| authorships[29].raw_author_name | Amanathan Kirthivasan |
| authorships[29].is_corresponding | False |
| authorships[29].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[30].author.id | https://openalex.org/A5101205554 |
| authorships[30].author.orcid | |
| authorships[30].author.display_name | Anwar Ijaz |
| authorships[30].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[30].author_position | middle |
| authorships[30].raw_author_name | Anwar Ijaz |
| authorships[30].is_corresponding | False |
| authorships[30].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[31].author.id | https://openalex.org/A5109405259 |
| authorships[31].author.orcid | https://orcid.org/0000-0002-6733-6938 |
| authorships[31].author.display_name | Kirsty Harkness |
| authorships[31].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[31].author_position | middle |
| authorships[31].raw_author_name | Kirsty Harkness |
| authorships[31].is_corresponding | False |
| authorships[31].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[32].author.id | https://openalex.org/A5014990756 |
| authorships[32].author.orcid | |
| authorships[32].author.display_name | Sevasti Ispoglou |
| authorships[32].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[32].author_position | middle |
| authorships[32].raw_author_name | Sevasti Ispoglou |
| authorships[32].is_corresponding | False |
| authorships[32].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[33].author.id | https://openalex.org/A5044234074 |
| authorships[33].author.orcid | |
| authorships[33].author.display_name | Nigel Smyth |
| authorships[33].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[33].author_position | middle |
| authorships[33].raw_author_name | Nigel Smyth |
| authorships[33].is_corresponding | False |
| authorships[33].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[34].author.id | https://openalex.org/A5072276293 |
| authorships[34].author.orcid | https://orcid.org/0000-0002-3848-0009 |
| authorships[34].author.display_name | Aravinth Sivagnanaratnam |
| authorships[34].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[34].author_position | middle |
| authorships[34].raw_author_name | Aravinth Sivagnanaratnam |
| authorships[34].is_corresponding | False |
| authorships[34].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[35].author.id | https://openalex.org/A5101724076 |
| authorships[35].author.orcid | https://orcid.org/0000-0001-7318-8567 |
| authorships[35].author.display_name | David Cohen |
| authorships[35].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[35].author_position | middle |
| authorships[35].raw_author_name | David Cohen |
| authorships[35].is_corresponding | False |
| authorships[35].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[36].author.id | https://openalex.org/A5063877776 |
| authorships[36].author.orcid | https://orcid.org/0009-0005-8037-6917 |
| authorships[36].author.display_name | Lakshmanan Sekaran |
| authorships[36].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[36].author_position | middle |
| authorships[36].raw_author_name | Lakshmanan Sekaran |
| authorships[36].is_corresponding | False |
| authorships[36].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[37].author.id | https://openalex.org/A5063821824 |
| authorships[37].author.orcid | |
| authorships[37].author.display_name | Dinesh Chadha |
| authorships[37].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[37].author_position | middle |
| authorships[37].raw_author_name | Dinesh Chadha |
| authorships[37].is_corresponding | False |
| authorships[37].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[38].author.id | https://openalex.org/A5100570924 |
| authorships[38].author.orcid | |
| authorships[38].author.display_name | Nasar Ahmad |
| authorships[38].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[38].author_position | middle |
| authorships[38].raw_author_name | Nasar Ahmad |
| authorships[38].is_corresponding | False |
| authorships[38].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[39].author.id | https://openalex.org/A5103848904 |
| authorships[39].author.orcid | |
| authorships[39].author.display_name | Pratap Rana |
| authorships[39].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[39].author_position | middle |
| authorships[39].raw_author_name | Pratap Rana |
| authorships[39].is_corresponding | False |
| authorships[39].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[40].author.id | https://openalex.org/A5015532179 |
| authorships[40].author.orcid | |
| authorships[40].author.display_name | Malik Azhar Hussain |
| authorships[40].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[40].author_position | middle |
| authorships[40].raw_author_name | Malik Hussain |
| authorships[40].is_corresponding | False |
| authorships[40].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[41].author.id | https://openalex.org/A5033786861 |
| authorships[41].author.orcid | |
| authorships[41].author.display_name | Nic Weir |
| authorships[41].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[41].author_position | middle |
| authorships[41].raw_author_name | Nic Weir |
| authorships[41].is_corresponding | False |
| authorships[41].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[42].author.id | https://openalex.org/A5034898456 |
| authorships[42].author.orcid | https://orcid.org/0000-0003-3619-4348 |
| authorships[42].author.display_name | Thomas S. Harrison |
| authorships[42].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[42].author_position | middle |
| authorships[42].raw_author_name | Thomas Harrison |
| authorships[42].is_corresponding | False |
| authorships[42].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[43].author.id | https://openalex.org/A5080266840 |
| authorships[43].author.orcid | https://orcid.org/0000-0002-0303-720X |
| authorships[43].author.display_name | Salim Elyas |
| authorships[43].affiliations[0].raw_affiliation_string | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| authorships[43].author_position | last |
| authorships[43].raw_author_name | Salim Elyas |
| authorships[43].is_corresponding | False |
| authorships[43].raw_affiliation_strings | for the Lacunar Intervention Trial-2 (LACI-2) Investigator Group |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdf |
| open_access.oa_status | hybrid |
| open_access.any_repository_has_fulltext | False |
| created_date | 2023-05-25T00:00:00 |
| display_name | Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10227 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9993000030517578 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2713 |
| primary_topic.subfield.display_name | Epidemiology |
| primary_topic.display_name | Acute Ischemic Stroke Management |
| related_works | https://openalex.org/W4394680473, https://openalex.org/W2050054037, https://openalex.org/W4380356744, https://openalex.org/W4319063241, https://openalex.org/W2994338014, https://openalex.org/W4387889430, https://openalex.org/W2116048257, https://openalex.org/W2625185915, https://openalex.org/W1991162655, https://openalex.org/W1480879219 |
| cited_by_count | 101 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 38 |
| counts_by_year[1].year | 2024 |
| counts_by_year[1].cited_by_count | 46 |
| counts_by_year[2].year | 2023 |
| counts_by_year[2].cited_by_count | 17 |
| locations_count | 6 |
| best_oa_location.id | doi:10.1001/jamaneurol.2023.1526 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S164389565 |
| best_oa_location.source.issn | 2168-6149, 2168-6157 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | 2168-6149 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | JAMA Neurology |
| best_oa_location.source.host_organization | https://openalex.org/P4310320259 |
| best_oa_location.source.host_organization_name | American Medical Association |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320259 |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | JAMA Neurology |
| best_oa_location.landing_page_url | https://doi.org/10.1001/jamaneurol.2023.1526 |
| primary_location.id | doi:10.1001/jamaneurol.2023.1526 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S164389565 |
| primary_location.source.issn | 2168-6149, 2168-6157 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 2168-6149 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | JAMA Neurology |
| primary_location.source.host_organization | https://openalex.org/P4310320259 |
| primary_location.source.host_organization_name | American Medical Association |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320259 |
| primary_location.license | cc-by |
| primary_location.pdf_url | https://jamanetwork.com/journals/jamaneurology/articlepdf/2805321/jamaneurology_wardlaw_2023_oi_230032_1684339623.44286.pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | JAMA Neurology |
| primary_location.landing_page_url | https://doi.org/10.1001/jamaneurol.2023.1526 |
| publication_date | 2023-05-24 |
| publication_year | 2023 |
| referenced_works | https://openalex.org/W3127198243, https://openalex.org/W2946066695, https://openalex.org/W2991513719, https://openalex.org/W3161563824, https://openalex.org/W2099852184, https://openalex.org/W4221096488, https://openalex.org/W2169070397, https://openalex.org/W3018172497, https://openalex.org/W3039038417, https://openalex.org/W6838965080, https://openalex.org/W2049072320, https://openalex.org/W2213019825, https://openalex.org/W2158742097, https://openalex.org/W2941825896, https://openalex.org/W3089635599, https://openalex.org/W2779726076, https://openalex.org/W2101091728, https://openalex.org/W3210362538, https://openalex.org/W2904618326, https://openalex.org/W2130167936, https://openalex.org/W2174394664, https://openalex.org/W2898320157, https://openalex.org/W4213172209, https://openalex.org/W2913926301, https://openalex.org/W2998714532, https://openalex.org/W2127624569, https://openalex.org/W2912533302, https://openalex.org/W2967318538, https://openalex.org/W4281751054, https://openalex.org/W4294275394 |
| referenced_works_count | 30 |
| abstract_inverted_index.2 | 82, 84 |
| abstract_inverted_index.3 | 499 |
| abstract_inverted_index.= | 309, 321, 350, 366, 382, 397, 408, 422, 437 |
| abstract_inverted_index.A | 260 |
| abstract_inverted_index.P | 308, 320, 349, 365, 381, 396, 407, 421, 436 |
| abstract_inverted_index.a | 7, 81 |
| abstract_inverted_index.12 | 193, 272 |
| abstract_inverted_index.26 | 95 |
| abstract_inverted_index.30 | 124 |
| abstract_inverted_index.5, | 102 |
| abstract_inverted_index.64 | 240 |
| abstract_inverted_index.79 | 256 |
| abstract_inverted_index.Of | 224 |
| abstract_inverted_index.To | 34 |
| abstract_inverted_index.UK | 96 |
| abstract_inverted_index.an | 72 |
| abstract_inverted_index.at | 192, 271 |
| abstract_inverted_index.be | 494 |
| abstract_inverted_index.in | 57, 268, 328, 336, 355, 371, 439, 488, 496 |
| abstract_inverted_index.is | 6, 14 |
| abstract_inverted_index.no | 30, 137, 179, 444 |
| abstract_inverted_index.of | 10, 19, 43, 202, 218, 262, 276, 283 |
| abstract_inverted_index.on | 51, 150 |
| abstract_inverted_index.or | 178, 281 |
| abstract_inverted_index.to | 90, 133, 139, 165, 306, 318, 347, 363, 379, 394, 405, 419, 434 |
| abstract_inverted_index.× | 83 |
| abstract_inverted_index.(or | 140 |
| abstract_inverted_index.12, | 152 |
| abstract_inverted_index.153 | 440 |
| abstract_inverted_index.200 | 175 |
| abstract_inverted_index.251 | 244 |
| abstract_inverted_index.257 | 275 |
| abstract_inverted_index.272 | 277 |
| abstract_inverted_index.297 | 329 |
| abstract_inverted_index.308 | 356 |
| abstract_inverted_index.31, | 106 |
| abstract_inverted_index.320 | 372 |
| abstract_inverted_index.353 | 337 |
| abstract_inverted_index.358 | 263 |
| abstract_inverted_index.363 | 232 |
| abstract_inverted_index.400 | 92, 226 |
| abstract_inverted_index.50% | 280 |
| abstract_inverted_index.All | 155 |
| abstract_inverted_index.CI, | 304, 316, 345, 361, 377, 392, 403, 417, 432 |
| abstract_inverted_index.May | 105 |
| abstract_inverted_index.QOL | 426 |
| abstract_inverted_index.The | 66, 87, 184, 248 |
| abstract_inverted_index.age | 238 |
| abstract_inverted_index.and | 23, 26, 41, 48, 54, 64, 104, 135, 162, 174, 207, 221, 253, 352, 410, 424, 448, 459, 461, 466, 475, 481 |
| abstract_inverted_index.any | 411 |
| abstract_inverted_index.but | 28 |
| abstract_inverted_index.for | 229 |
| abstract_inverted_index.had | 113, 126, 131, 136 |
| abstract_inverted_index.has | 29 |
| abstract_inverted_index.may | 470 |
| abstract_inverted_index.nor | 311 |
| abstract_inverted_index.not | 291 |
| abstract_inverted_index.the | 15, 36, 143, 225, 269, 284, 325, 387, 454 |
| abstract_inverted_index.was | 71, 148, 187, 239, 255, 457 |
| abstract_inverted_index.(200 | 170 |
| abstract_inverted_index..01) | 351 |
| abstract_inverted_index..02) | 423 |
| abstract_inverted_index..10) | 322 |
| abstract_inverted_index..16) | 310 |
| abstract_inverted_index.0.03 | 404, 433 |
| abstract_inverted_index.0.07 | 346 |
| abstract_inverted_index.0.10 | 430 |
| abstract_inverted_index.0.14 | 378, 401 |
| abstract_inverted_index.0.23 | 343, 418 |
| abstract_inverted_index.0.31 | 375 |
| abstract_inverted_index.0.36 | 362, 393 |
| abstract_inverted_index.0.44 | 415 |
| abstract_inverted_index.0.55 | 359 |
| abstract_inverted_index.0.57 | 317 |
| abstract_inverted_index.0.58 | 390 |
| abstract_inverted_index.0.59 | 305 |
| abstract_inverted_index.0.77 | 314 |
| abstract_inverted_index.0.80 | 302 |
| abstract_inverted_index.Data | 146 |
| abstract_inverted_index.ISMN | 166, 297, 460 |
| abstract_inverted_index.Main | 182 |
| abstract_inverted_index.[95% | 303, 315, 344, 360, 376, 391, 402, 416, 431 |
| abstract_inverted_index.aged | 121 |
| abstract_inverted_index.both | 491 |
| abstract_inverted_index.drug | 38, 209 |
| abstract_inverted_index.for) | 142 |
| abstract_inverted_index.from | 94 |
| abstract_inverted_index.life | 219 |
| abstract_inverted_index.mean | 428 |
| abstract_inverted_index.men. | 247 |
| abstract_inverted_index.mood | 27 |
| abstract_inverted_index.more | 282 |
| abstract_inverted_index.most | 16 |
| abstract_inverted_index.odds | 340 |
| abstract_inverted_index.show | 452 |
| abstract_inverted_index.test | 35 |
| abstract_inverted_index.than | 123 |
| abstract_inverted_index.that | 293, 453 |
| abstract_inverted_index.they | 482 |
| abstract_inverted_index.this | 230 |
| abstract_inverted_index.time | 250 |
| abstract_inverted_index.well | 464 |
| abstract_inverted_index.were | 118, 120, 163, 197, 234, 246, 266, 443, 463 |
| abstract_inverted_index.with | 59, 80, 108, 274, 288 |
| abstract_inverted_index.(IQR, | 241, 257 |
| abstract_inverted_index.(aHR, | 313, 374, 389 |
| abstract_inverted_index.(aOR, | 358, 400, 414 |
| abstract_inverted_index..005) | 438 |
| abstract_inverted_index..02), | 398 |
| abstract_inverted_index.2018, | 103 |
| abstract_inverted_index.2021, | 107 |
| abstract_inverted_index.2022. | 153 |
| abstract_inverted_index.Their | 236 |
| abstract_inverted_index.There | 442 |
| abstract_inverted_index.These | 450, 468 |
| abstract_inverted_index.Trial | 501 |
| abstract_inverted_index.after | 478 |
| abstract_inverted_index.aimed | 89 |
| abstract_inverted_index.alone | 323 |
| abstract_inverted_index.brain | 128 |
| abstract_inverted_index.cSVD. | 489 |
| abstract_inverted_index.cause | 9, 18 |
| abstract_inverted_index.could | 483 |
| abstract_inverted_index.days. | 259 |
| abstract_inverted_index.drug, | 295 |
| abstract_inverted_index.drug. | 181, 286 |
| abstract_inverted_index.large | 497 |
| abstract_inverted_index.mg/d, | 176 |
| abstract_inverted_index.older | 122 |
| abstract_inverted_index.other | 485 |
| abstract_inverted_index.phase | 498 |
| abstract_inverted_index.ratio | 300, 341 |
| abstract_inverted_index.safe. | 467 |
| abstract_inverted_index.small | 2 |
| abstract_inverted_index.study | 144, 180, 270 |
| abstract_inverted_index.those | 289 |
| abstract_inverted_index.total | 261 |
| abstract_inverted_index.trial | 79, 88, 456 |
| abstract_inverted_index.(40-60 | 167, 173 |
| abstract_inverted_index.(ISMN) | 47 |
| abstract_inverted_index.(QOL), | 220 |
| abstract_inverted_index.(cSVD) | 5 |
| abstract_inverted_index..006). | 383 |
| abstract_inverted_index..008), | 409 |
| abstract_inverted_index..008). | 367 |
| abstract_inverted_index.0.17]; | 435 |
| abstract_inverted_index.0.59]; | 406 |
| abstract_inverted_index.0.72]; | 380 |
| abstract_inverted_index.0.74]; | 348 |
| abstract_inverted_index.0.85]; | 420 |
| abstract_inverted_index.0.86]; | 364 |
| abstract_inverted_index.0.92]; | 395 |
| abstract_inverted_index.1-year | 44 |
| abstract_inverted_index.1.05]; | 319 |
| abstract_inverted_index.1.09]; | 307 |
| abstract_inverted_index.August | 151 |
| abstract_inverted_index.LACI-2 | 455 |
| abstract_inverted_index.[aHR], | 301 |
| abstract_inverted_index.[aOR], | 342 |
| abstract_inverted_index.agents | 469, 492 |
| abstract_inverted_index.common | 8, 17 |
| abstract_inverted_index.drugs. | 145 |
| abstract_inverted_index.hazard | 299 |
| abstract_inverted_index.median | 237, 249 |
| abstract_inverted_index.mg/d), | 168, 171 |
| abstract_inverted_index.reduce | 471 |
| abstract_inverted_index.safety | 198, 445 |
| abstract_inverted_index.should | 493 |
| abstract_inverted_index.stroke | 11, 98, 159, 252, 335 |
| abstract_inverted_index.taking | 279 |
| abstract_inverted_index.tested | 495 |
| abstract_inverted_index.trial, | 231 |
| abstract_inverted_index.vessel | 3 |
| abstract_inverted_index.years, | 125 |
| abstract_inverted_index.years; | 243 |
| abstract_inverted_index.(69.1%) | 245 |
| abstract_inverted_index.(90.8%) | 233 |
| abstract_inverted_index.(94.5%) | 278 |
| abstract_inverted_index.(98.6%) | 265 |
| abstract_inverted_index.Design, | 62 |
| abstract_inverted_index.Lacunar | 67 |
| abstract_inverted_index.Results | 223 |
| abstract_inverted_index.Trial-2 | 69 |
| abstract_inverted_index.adverse | 486 |
| abstract_inverted_index.between | 100, 251 |
| abstract_inverted_index.blinded | 75 |
| abstract_inverted_index.centers | 99 |
| abstract_inverted_index.death), | 208 |
| abstract_inverted_index.design. | 86 |
| abstract_inverted_index.disease | 4 |
| abstract_inverted_index.effects | 42 |
| abstract_inverted_index.events, | 204 |
| abstract_inverted_index.imaging | 129 |
| abstract_inverted_index.impairs | 24 |
| abstract_inverted_index.lacunar | 60, 115, 479 |
| abstract_inverted_index.months, | 273 |
| abstract_inverted_index.months. | 194 |
| abstract_inverted_index.neither | 296 |
| abstract_inverted_index.outcome | 186, 327 |
| abstract_inverted_index.planned | 228 |
| abstract_inverted_index.prevent | 484 |
| abstract_inverted_index.primary | 185 |
| abstract_inverted_index.quality | 217 |
| abstract_inverted_index.recruit | 91 |
| abstract_inverted_index.reduced | 324, 333, 369, 386 |
| abstract_inverted_index.results | 451 |
| abstract_inverted_index.safety, | 40 |
| abstract_inverted_index.stroke, | 117, 213, 473, 480 |
| abstract_inverted_index.stroke. | 61 |
| abstract_inverted_index.trials. | 500 |
| abstract_inverted_index.(LACI-2) | 70 |
| abstract_inverted_index.(death), | 199 |
| abstract_inverted_index.(lacunar | 12 |
| abstract_inverted_index.12-month | 109 |
| abstract_inverted_index.Cerebral | 1 |
| abstract_inverted_index.Compared | 287 |
| abstract_inverted_index.February | 101 |
| abstract_inverted_index.Included | 111 |
| abstract_inverted_index.Outcomes | 183 |
| abstract_inverted_index.Setting, | 63 |
| abstract_inverted_index.analysis | 147 |
| abstract_inverted_index.capacity | 132 |
| abstract_inverted_index.clinical | 78, 114 |
| abstract_inverted_index.consent, | 134 |
| abstract_inverted_index.efficacy | 200 |
| abstract_inverted_index.feasible | 458 |
| abstract_inverted_index.hospital | 97 |
| abstract_inverted_index.improved | 425 |
| abstract_inverted_index.ischemic | 116 |
| abstract_inverted_index.mobility | 25 |
| abstract_inverted_index.outcomes | 56, 196, 487 |
| abstract_inverted_index.patients | 58, 156, 264, 338, 357, 373 |
| abstract_inverted_index.received | 157 |
| abstract_inverted_index.retained | 267 |
| abstract_inverted_index.specific | 31 |
| abstract_inverted_index.stroke), | 13 |
| abstract_inverted_index.vascular | 20, 203 |
| abstract_inverted_index.(adjusted | 298, 339, 427 |
| abstract_inverted_index.Objective | 33 |
| abstract_inverted_index.Relevance | 449 |
| abstract_inverted_index.Secondary | 195 |
| abstract_inverted_index.allocated | 285 |
| abstract_inverted_index.cognitive | 21, 55, 215, 353, 412, 476 |
| abstract_inverted_index.composite | 326, 388 |
| abstract_inverted_index.concerns. | 446 |
| abstract_inverted_index.factorial | 85 |
| abstract_inverted_index.findings, | 130 |
| abstract_inverted_index.guideline | 158 |
| abstract_inverted_index.including | 190 |
| abstract_inverted_index.patients. | 330, 441 |
| abstract_inverted_index.performed | 149 |
| abstract_inverted_index.receiving | 292 |
| abstract_inverted_index.recurrent | 212, 334, 472 |
| abstract_inverted_index.retention | 191 |
| abstract_inverted_index.tolerated | 465 |
| abstract_inverted_index.treatment | 50, 161 |
| abstract_inverted_index.vascular, | 52 |
| abstract_inverted_index.(composite | 201 |
| abstract_inverted_index.56.0-72.0) | 242 |
| abstract_inverted_index.Cilostazol | 368 |
| abstract_inverted_index.Importance | 0 |
| abstract_inverted_index.Isosorbide | 331 |
| abstract_inverted_index.Therefore, | 490 |
| abstract_inverted_index.adherence, | 210 |
| abstract_inverted_index.cilostazol | 49, 169, 312, 462 |
| abstract_inverted_index.cognition, | 206 |
| abstract_inverted_index.compatible | 127 |
| abstract_inverted_index.dependence | 370, 399 |
| abstract_inverted_index.end-point, | 76 |
| abstract_inverted_index.follow-up. | 110 |
| abstract_inverted_index.impairment | 354, 413, 477 |
| abstract_inverted_index.isosorbide | 45 |
| abstract_inverted_index.particular | 294 |
| abstract_inverted_index.prevention | 160 |
| abstract_inverted_index.randomized | 77, 164 |
| abstract_inverted_index.recruited. | 235 |
| abstract_inverted_index.treatment. | 32 |
| abstract_inverted_index.27.0-244.0) | 258 |
| abstract_inverted_index.Combination | 384 |
| abstract_inverted_index.Conclusions | 447 |
| abstract_inverted_index.Identifier: | 504 |
| abstract_inverted_index.NCT03451591 | 505 |
| abstract_inverted_index.dependence, | 205, 214, 474 |
| abstract_inverted_index.difference, | 429 |
| abstract_inverted_index.functional, | 53 |
| abstract_inverted_index.hemorrhage. | 222 |
| abstract_inverted_index.impairment, | 22, 216 |
| abstract_inverted_index.indications | 141 |
| abstract_inverted_index.mononitrate | 46, 332 |
| abstract_inverted_index.open-label, | 74 |
| abstract_inverted_index.recruitment | 188 |
| abstract_inverted_index.Intervention | 68 |
| abstract_inverted_index.Participants | 65 |
| abstract_inverted_index.Registration | 502 |
| abstract_inverted_index.feasibility, | 37, 189 |
| abstract_inverted_index.independent, | 119 |
| abstract_inverted_index.participants | 93, 112, 227, 290 |
| abstract_inverted_index.Interventions | 154 |
| abstract_inverted_index.randomization | 254 |
| abstract_inverted_index.tolerability, | 39, 211 |
| abstract_inverted_index.respectively), | 177 |
| abstract_inverted_index.ISMN-cilostazol | 172, 385 |
| abstract_inverted_index.contraindications | 138 |
| abstract_inverted_index.ClinicalTrials.gov | 503 |
| abstract_inverted_index.investigator-initiated, | 73 |
| cited_by_percentile_year.max | 100 |
| cited_by_percentile_year.min | 99 |
| corresponding_author_ids | https://openalex.org/A5004889443 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 44 |
| corresponding_institution_ids | https://openalex.org/I4210126463, https://openalex.org/I98677209 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.8299999833106995 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.99746728 |
| citation_normalized_percentile.is_in_top_1_percent | True |
| citation_normalized_percentile.is_in_top_10_percent | True |