Light-EnhancedGas Selectivity in SnO2–xFy Nanocrystals: CompetitiveAdsorption Driven by Carbon Chain Length, Isomerism, and FunctionalGroups Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1021/acssensors.5c01504.s004
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1021/acssensors.5c01504.s004
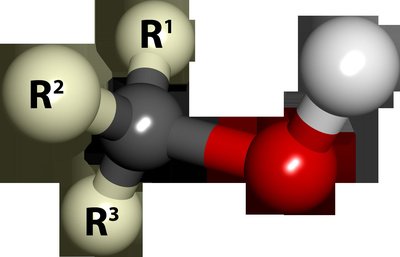
An attempt was made to explore the essence of gas selectivity and the miscellaneous reactive sites of stannic oxide, which were obtained by combining oxygen reduction with increasing fluorine and activated by light irradiation. The microstructure and gas-sensing performance of stannic oxide were analyzed using X-ray diffraction, Raman spectroscopy, atomic force microscopy, ultraviolet spectrophotometry, and an electrochemical testing system. The results show that the carbon chain length, functional group position, and functional group species of the measured gas determine the gas-sensing performance of the nanocrystalline SnO2–xFy precipitated from quaternary Zn–O–F–Sn amorphous films by designing the injection sequence of gas in time and space. For 100 ppm gas molecules, the gas response sequences of the SnO2–xFy films are methanol (42.57) > ethanol (30.14) > 1-propanol (15.63) > 2-propanol (12.36) > acetone (10.32). The alcohol gases are apt to be adsorbed on oxygen vacancies to reflect the higher response value and higher optimal temperatures (280 °C), while ketone gases prefer to be adsorbed at the active site produced by fluorine to reflect fast response/recovery speed and lower working temperatures (260 °C).
Related Topics
- Type
- article
- OA Status
- green
- OpenAlex ID
- https://openalex.org/W7110799271
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W7110799271Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1021/acssensors.5c01504.s004Digital Object Identifier
- Title
-
Light-EnhancedGas Selectivity in SnO2–xFy Nanocrystals: CompetitiveAdsorption Driven by Carbon Chain Length, Isomerism, and FunctionalGroupsWork title
- Type
-
articleOpenAlex work type
- Publication year
-
2025Year of publication
- Publication date
-
2025-10-30Full publication date if available
- Authors
-
Zheng-yun Tian (22529274), Hu-wei Miao (22529277), Jin-chen Yao (22529280), Qing-ru Liu (22529283), Wei Liu (20030), Li-qing Bu (22529286), Jin-shan Chen (22529289), Jun-cai Wu (22529292), Hao-yu Jiang (22529295), Fa-yu Wu (22529298), Yong-xing Liu (22529301)List of authors in order
- Open access
-
YesWhether a free full text is available
- OA status
-
greenOpen access status per OpenAlex
- Concepts
-
Chemistry, Adsorption, Selectivity, Ketone, Methanol, Oxygen, Fluorine, Alcohol, Nanocrystalline material, Inorganic chemistry, Acetone, Functional group, Raman spectroscopy, Carbon fibers, Ethanol, Amorphous solid, Carbon black, Carbon monoxide, Oxide, Ultraviolet light, Microstructure, Electrochemistry, Methylene, Partial oxidation, Calcium oxide, Chemical engineering, Organic chemistry, Methane, Formaldehyde, Amorphous carbon, Ultraviolet, Cationic polymerization, Activated carbon, Primary alcoholTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
Full payload
| id | https://openalex.org/W7110799271 |
|---|---|
| doi | https://doi.org/10.1021/acssensors.5c01504.s004 |
| ids.openalex | https://openalex.org/W7110799271 |
| fwci | 0.0 |
| type | article |
| title | Light-EnhancedGas Selectivity in SnO2–xFy Nanocrystals: CompetitiveAdsorption Driven by Carbon Chain Length, Isomerism, and FunctionalGroups |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | |
| biblio.first_page | |
| topics[0].id | https://openalex.org/T10461 |
| topics[0].field.id | https://openalex.org/fields/22 |
| topics[0].field.display_name | Engineering |
| topics[0].score | 0.9168791174888611 |
| topics[0].domain.id | https://openalex.org/domains/3 |
| topics[0].domain.display_name | Physical Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2208 |
| topics[0].subfield.display_name | Electrical and Electronic Engineering |
| topics[0].display_name | Gas Sensing Nanomaterials and Sensors |
| topics[1].id | https://openalex.org/T10090 |
| topics[1].field.id | https://openalex.org/fields/25 |
| topics[1].field.display_name | Materials Science |
| topics[1].score | 0.0538448765873909 |
| topics[1].domain.id | https://openalex.org/domains/3 |
| topics[1].domain.display_name | Physical Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2505 |
| topics[1].subfield.display_name | Materials Chemistry |
| topics[1].display_name | ZnO doping and properties |
| topics[2].id | https://openalex.org/T12529 |
| topics[2].field.id | https://openalex.org/fields/25 |
| topics[2].field.display_name | Materials Science |
| topics[2].score | 0.004462132696062326 |
| topics[2].domain.id | https://openalex.org/domains/3 |
| topics[2].domain.display_name | Physical Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2504 |
| topics[2].subfield.display_name | Electronic, Optical and Magnetic Materials |
| topics[2].display_name | Ga2O3 and related materials |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C185592680 |
| concepts[0].level | 0 |
| concepts[0].score | 0.6939230561256409 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q2329 |
| concepts[0].display_name | Chemistry |
| concepts[1].id | https://openalex.org/C150394285 |
| concepts[1].level | 2 |
| concepts[1].score | 0.6847527623176575 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q180254 |
| concepts[1].display_name | Adsorption |
| concepts[2].id | https://openalex.org/C118792377 |
| concepts[2].level | 3 |
| concepts[2].score | 0.6070905327796936 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q108584245 |
| concepts[2].display_name | Selectivity |
| concepts[3].id | https://openalex.org/C2777738585 |
| concepts[3].level | 2 |
| concepts[3].score | 0.5955286026000977 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q170744 |
| concepts[3].display_name | Ketone |
| concepts[4].id | https://openalex.org/C2779607525 |
| concepts[4].level | 2 |
| concepts[4].score | 0.5945965647697449 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q14982 |
| concepts[4].display_name | Methanol |
| concepts[5].id | https://openalex.org/C540031477 |
| concepts[5].level | 2 |
| concepts[5].score | 0.5784116387367249 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q629 |
| concepts[5].display_name | Oxygen |
| concepts[6].id | https://openalex.org/C506198293 |
| concepts[6].level | 2 |
| concepts[6].score | 0.5451731085777283 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q650 |
| concepts[6].display_name | Fluorine |
| concepts[7].id | https://openalex.org/C2781066024 |
| concepts[7].level | 2 |
| concepts[7].score | 0.5370575189590454 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q156 |
| concepts[7].display_name | Alcohol |
| concepts[8].id | https://openalex.org/C140676511 |
| concepts[8].level | 2 |
| concepts[8].score | 0.525276780128479 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q6964018 |
| concepts[8].display_name | Nanocrystalline material |
| concepts[9].id | https://openalex.org/C179104552 |
| concepts[9].level | 1 |
| concepts[9].score | 0.5179614424705505 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q11165 |
| concepts[9].display_name | Inorganic chemistry |
| concepts[10].id | https://openalex.org/C2781213060 |
| concepts[10].level | 2 |
| concepts[10].score | 0.4762589633464813 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q49546 |
| concepts[10].display_name | Acetone |
| concepts[11].id | https://openalex.org/C2777332933 |
| concepts[11].level | 3 |
| concepts[11].score | 0.40796732902526855 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q170409 |
| concepts[11].display_name | Functional group |
| concepts[12].id | https://openalex.org/C40003534 |
| concepts[12].level | 2 |
| concepts[12].score | 0.40415042638778687 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q862228 |
| concepts[12].display_name | Raman spectroscopy |
| concepts[13].id | https://openalex.org/C140205800 |
| concepts[13].level | 3 |
| concepts[13].score | 0.3827989101409912 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q5860 |
| concepts[13].display_name | Carbon fibers |
| concepts[14].id | https://openalex.org/C2780161600 |
| concepts[14].level | 2 |
| concepts[14].score | 0.37875255942344666 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q153 |
| concepts[14].display_name | Ethanol |
| concepts[15].id | https://openalex.org/C56052488 |
| concepts[15].level | 2 |
| concepts[15].score | 0.3753741383552551 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q103382 |
| concepts[15].display_name | Amorphous solid |
| concepts[16].id | https://openalex.org/C44038986 |
| concepts[16].level | 3 |
| concepts[16].score | 0.36942794919013977 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q764245 |
| concepts[16].display_name | Carbon black |
| concepts[17].id | https://openalex.org/C512735826 |
| concepts[17].level | 3 |
| concepts[17].score | 0.3675123453140259 |
| concepts[17].wikidata | https://www.wikidata.org/wiki/Q2025 |
| concepts[17].display_name | Carbon monoxide |
| concepts[18].id | https://openalex.org/C2779851234 |
| concepts[18].level | 2 |
| concepts[18].score | 0.3675001561641693 |
| concepts[18].wikidata | https://www.wikidata.org/wiki/Q50690 |
| concepts[18].display_name | Oxide |
| concepts[19].id | https://openalex.org/C2987116853 |
| concepts[19].level | 2 |
| concepts[19].score | 0.35806477069854736 |
| concepts[19].wikidata | https://www.wikidata.org/wiki/Q11391 |
| concepts[19].display_name | Ultraviolet light |
| concepts[20].id | https://openalex.org/C87976508 |
| concepts[20].level | 2 |
| concepts[20].score | 0.34282657504081726 |
| concepts[20].wikidata | https://www.wikidata.org/wiki/Q1498213 |
| concepts[20].display_name | Microstructure |
| concepts[21].id | https://openalex.org/C52859227 |
| concepts[21].level | 3 |
| concepts[21].score | 0.3335360288619995 |
| concepts[21].wikidata | https://www.wikidata.org/wiki/Q7877 |
| concepts[21].display_name | Electrochemistry |
| concepts[22].id | https://openalex.org/C2777454769 |
| concepts[22].level | 2 |
| concepts[22].score | 0.32736900448799133 |
| concepts[22].wikidata | https://www.wikidata.org/wiki/Q11172462 |
| concepts[22].display_name | Methylene |
| concepts[23].id | https://openalex.org/C110686534 |
| concepts[23].level | 3 |
| concepts[23].score | 0.31721869111061096 |
| concepts[23].wikidata | https://www.wikidata.org/wiki/Q451467 |
| concepts[23].display_name | Partial oxidation |
| concepts[24].id | https://openalex.org/C2777312818 |
| concepts[24].level | 2 |
| concepts[24].score | 0.3105059564113617 |
| concepts[24].wikidata | https://www.wikidata.org/wiki/Q185006 |
| concepts[24].display_name | Calcium oxide |
| concepts[25].id | https://openalex.org/C42360764 |
| concepts[25].level | 1 |
| concepts[25].score | 0.3045240342617035 |
| concepts[25].wikidata | https://www.wikidata.org/wiki/Q83588 |
| concepts[25].display_name | Chemical engineering |
| concepts[26].id | https://openalex.org/C178790620 |
| concepts[26].level | 1 |
| concepts[26].score | 0.30195555090904236 |
| concepts[26].wikidata | https://www.wikidata.org/wiki/Q11351 |
| concepts[26].display_name | Organic chemistry |
| concepts[27].id | https://openalex.org/C516920438 |
| concepts[27].level | 2 |
| concepts[27].score | 0.2960612177848816 |
| concepts[27].wikidata | https://www.wikidata.org/wiki/Q37129 |
| concepts[27].display_name | Methane |
| concepts[28].id | https://openalex.org/C2775930285 |
| concepts[28].level | 2 |
| concepts[28].score | 0.2946750521659851 |
| concepts[28].wikidata | https://www.wikidata.org/wiki/Q161210 |
| concepts[28].display_name | Formaldehyde |
| concepts[29].id | https://openalex.org/C201931942 |
| concepts[29].level | 3 |
| concepts[29].score | 0.27469930052757263 |
| concepts[29].wikidata | https://www.wikidata.org/wiki/Q798012 |
| concepts[29].display_name | Amorphous carbon |
| concepts[30].id | https://openalex.org/C2776798109 |
| concepts[30].level | 2 |
| concepts[30].score | 0.2720230221748352 |
| concepts[30].wikidata | https://www.wikidata.org/wiki/Q11391 |
| concepts[30].display_name | Ultraviolet |
| concepts[31].id | https://openalex.org/C183882617 |
| concepts[31].level | 2 |
| concepts[31].score | 0.2690984010696411 |
| concepts[31].wikidata | https://www.wikidata.org/wiki/Q3046749 |
| concepts[31].display_name | Cationic polymerization |
| concepts[32].id | https://openalex.org/C2779647737 |
| concepts[32].level | 3 |
| concepts[32].score | 0.26472461223602295 |
| concepts[32].wikidata | https://www.wikidata.org/wiki/Q190878 |
| concepts[32].display_name | Activated carbon |
| concepts[33].id | https://openalex.org/C2781327392 |
| concepts[33].level | 3 |
| concepts[33].score | 0.25357601046562195 |
| concepts[33].wikidata | https://www.wikidata.org/wiki/Q2832210 |
| concepts[33].display_name | Primary alcohol |
| keywords[0].id | https://openalex.org/keywords/adsorption |
| keywords[0].score | 0.6847527623176575 |
| keywords[0].display_name | Adsorption |
| keywords[1].id | https://openalex.org/keywords/selectivity |
| keywords[1].score | 0.6070905327796936 |
| keywords[1].display_name | Selectivity |
| keywords[2].id | https://openalex.org/keywords/ketone |
| keywords[2].score | 0.5955286026000977 |
| keywords[2].display_name | Ketone |
| keywords[3].id | https://openalex.org/keywords/methanol |
| keywords[3].score | 0.5945965647697449 |
| keywords[3].display_name | Methanol |
| keywords[4].id | https://openalex.org/keywords/oxygen |
| keywords[4].score | 0.5784116387367249 |
| keywords[4].display_name | Oxygen |
| keywords[5].id | https://openalex.org/keywords/fluorine |
| keywords[5].score | 0.5451731085777283 |
| keywords[5].display_name | Fluorine |
| keywords[6].id | https://openalex.org/keywords/alcohol |
| keywords[6].score | 0.5370575189590454 |
| keywords[6].display_name | Alcohol |
| keywords[7].id | https://openalex.org/keywords/nanocrystalline-material |
| keywords[7].score | 0.525276780128479 |
| keywords[7].display_name | Nanocrystalline material |
| keywords[8].id | https://openalex.org/keywords/acetone |
| keywords[8].score | 0.4762589633464813 |
| keywords[8].display_name | Acetone |
| keywords[9].id | https://openalex.org/keywords/functional-group |
| keywords[9].score | 0.40796732902526855 |
| keywords[9].display_name | Functional group |
| language | |
| locations[0].id | pmh:oai:figshare.com:article/30490530 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S4306400572 |
| locations[0].source.issn | |
| locations[0].source.type | repository |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | |
| locations[0].source.is_core | False |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | OPAL (Open@LaTrobe) (La Trobe University) |
| locations[0].source.host_organization | https://openalex.org/I196829312 |
| locations[0].source.host_organization_name | La Trobe University |
| locations[0].source.host_organization_lineage | https://openalex.org/I196829312 |
| locations[0].license | cc-by-nc |
| locations[0].pdf_url | |
| locations[0].version | submittedVersion |
| locations[0].raw_type | Journal contribution |
| locations[0].license_id | https://openalex.org/licenses/cc-by-nc |
| locations[0].is_accepted | False |
| locations[0].is_published | False |
| locations[0].raw_source_name | |
| locations[0].landing_page_url | |
| authorships[0].author.id | |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | Zheng-yun Tian (22529274) |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Zheng-yun Tian (22529274) |
| authorships[0].is_corresponding | True |
| authorships[1].author.id | |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Hu-wei Miao (22529277) |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Hu-wei Miao (22529277) |
| authorships[1].is_corresponding | False |
| authorships[2].author.id | |
| authorships[2].author.orcid | |
| authorships[2].author.display_name | Jin-chen Yao (22529280) |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Jin-chen Yao (22529280) |
| authorships[2].is_corresponding | False |
| authorships[3].author.id | |
| authorships[3].author.orcid | |
| authorships[3].author.display_name | Qing-ru Liu (22529283) |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Qing-ru Liu (22529283) |
| authorships[3].is_corresponding | False |
| authorships[4].author.id | |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Wei Liu (20030) |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Wei Liu (20030) |
| authorships[4].is_corresponding | False |
| authorships[5].author.id | |
| authorships[5].author.orcid | |
| authorships[5].author.display_name | Li-qing Bu (22529286) |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Li-qing Bu (22529286) |
| authorships[5].is_corresponding | False |
| authorships[6].author.id | |
| authorships[6].author.orcid | |
| authorships[6].author.display_name | Jin-shan Chen (22529289) |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Jin-shan Chen (22529289) |
| authorships[6].is_corresponding | False |
| authorships[7].author.id | |
| authorships[7].author.orcid | |
| authorships[7].author.display_name | Jun-cai Wu (22529292) |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Jun-cai Wu (22529292) |
| authorships[7].is_corresponding | False |
| authorships[8].author.id | |
| authorships[8].author.orcid | |
| authorships[8].author.display_name | Hao-yu Jiang (22529295) |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Hao-yu Jiang (22529295) |
| authorships[8].is_corresponding | False |
| authorships[9].author.id | |
| authorships[9].author.orcid | |
| authorships[9].author.display_name | Fa-yu Wu (22529298) |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Fa-yu Wu (22529298) |
| authorships[9].is_corresponding | False |
| authorships[10].author.id | |
| authorships[10].author.orcid | |
| authorships[10].author.display_name | Yong-xing Liu (22529301) |
| authorships[10].author_position | last |
| authorships[10].raw_author_name | Yong-xing Liu (22529301) |
| authorships[10].is_corresponding | False |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | |
| open_access.oa_status | green |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-12-10T00:00:00 |
| display_name | Light-EnhancedGas Selectivity in SnO2–xFy Nanocrystals: CompetitiveAdsorption Driven by Carbon Chain Length, Isomerism, and FunctionalGroups |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-12-10T02:49:46.989445 |
| primary_topic.id | https://openalex.org/T10461 |
| primary_topic.field.id | https://openalex.org/fields/22 |
| primary_topic.field.display_name | Engineering |
| primary_topic.score | 0.9168791174888611 |
| primary_topic.domain.id | https://openalex.org/domains/3 |
| primary_topic.domain.display_name | Physical Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2208 |
| primary_topic.subfield.display_name | Electrical and Electronic Engineering |
| primary_topic.display_name | Gas Sensing Nanomaterials and Sensors |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | pmh:oai:figshare.com:article/30490530 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S4306400572 |
| best_oa_location.source.issn | |
| best_oa_location.source.type | repository |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | |
| best_oa_location.source.is_core | False |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | OPAL (Open@LaTrobe) (La Trobe University) |
| best_oa_location.source.host_organization | https://openalex.org/I196829312 |
| best_oa_location.source.host_organization_name | La Trobe University |
| best_oa_location.source.host_organization_lineage | https://openalex.org/I196829312 |
| best_oa_location.license | cc-by-nc |
| best_oa_location.pdf_url | |
| best_oa_location.version | submittedVersion |
| best_oa_location.raw_type | Journal contribution |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by-nc |
| best_oa_location.is_accepted | False |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | |
| best_oa_location.landing_page_url | |
| primary_location.id | pmh:oai:figshare.com:article/30490530 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S4306400572 |
| primary_location.source.issn | |
| primary_location.source.type | repository |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | |
| primary_location.source.is_core | False |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | OPAL (Open@LaTrobe) (La Trobe University) |
| primary_location.source.host_organization | https://openalex.org/I196829312 |
| primary_location.source.host_organization_name | La Trobe University |
| primary_location.source.host_organization_lineage | https://openalex.org/I196829312 |
| primary_location.license | cc-by-nc |
| primary_location.pdf_url | |
| primary_location.version | submittedVersion |
| primary_location.raw_type | Journal contribution |
| primary_location.license_id | https://openalex.org/licenses/cc-by-nc |
| primary_location.is_accepted | False |
| primary_location.is_published | False |
| primary_location.raw_source_name | |
| primary_location.landing_page_url | |
| publication_date | 2025-10-30 |
| publication_year | 2025 |
| referenced_works_count | 0 |
| abstract_inverted_index.> | 119, 122, 125, 128 |
| abstract_inverted_index.An | 0 |
| abstract_inverted_index.an | 55 |
| abstract_inverted_index.at | 161 |
| abstract_inverted_index.be | 137, 159 |
| abstract_inverted_index.by | 22, 31, 92, 166 |
| abstract_inverted_index.in | 99 |
| abstract_inverted_index.of | 8, 16, 39, 74, 82, 97, 112 |
| abstract_inverted_index.on | 139 |
| abstract_inverted_index.to | 4, 136, 142, 158, 168 |
| abstract_inverted_index.100 | 104 |
| abstract_inverted_index.For | 103 |
| abstract_inverted_index.The | 34, 59, 131 |
| abstract_inverted_index.and | 11, 29, 36, 54, 70, 101, 148, 173 |
| abstract_inverted_index.apt | 135 |
| abstract_inverted_index.are | 116, 134 |
| abstract_inverted_index.gas | 9, 77, 98, 106, 109 |
| abstract_inverted_index.ppm | 105 |
| abstract_inverted_index.the | 6, 12, 63, 75, 79, 83, 94, 108, 113, 144, 162 |
| abstract_inverted_index.was | 2 |
| abstract_inverted_index.(260 | 177 |
| abstract_inverted_index.(280 | 152 |
| abstract_inverted_index.fast | 170 |
| abstract_inverted_index.from | 87 |
| abstract_inverted_index.made | 3 |
| abstract_inverted_index.show | 61 |
| abstract_inverted_index.site | 164 |
| abstract_inverted_index.that | 62 |
| abstract_inverted_index.time | 100 |
| abstract_inverted_index.were | 20, 42 |
| abstract_inverted_index.with | 26 |
| abstract_inverted_index.Raman | 47 |
| abstract_inverted_index.X-ray | 45 |
| abstract_inverted_index.chain | 65 |
| abstract_inverted_index.films | 91, 115 |
| abstract_inverted_index.force | 50 |
| abstract_inverted_index.gases | 133, 156 |
| abstract_inverted_index.group | 68, 72 |
| abstract_inverted_index.light | 32 |
| abstract_inverted_index.lower | 174 |
| abstract_inverted_index.oxide | 41 |
| abstract_inverted_index.sites | 15 |
| abstract_inverted_index.speed | 172 |
| abstract_inverted_index.using | 44 |
| abstract_inverted_index.value | 147 |
| abstract_inverted_index.which | 19 |
| abstract_inverted_index.while | 154 |
| abstract_inverted_index.°C), | 153 |
| abstract_inverted_index.°C). | 178 |
| abstract_inverted_index.active | 163 |
| abstract_inverted_index.atomic | 49 |
| abstract_inverted_index.carbon | 64 |
| abstract_inverted_index.higher | 145, 149 |
| abstract_inverted_index.ketone | 155 |
| abstract_inverted_index.oxide, | 18 |
| abstract_inverted_index.oxygen | 24, 140 |
| abstract_inverted_index.prefer | 157 |
| abstract_inverted_index.space. | 102 |
| abstract_inverted_index.(12.36) | 127 |
| abstract_inverted_index.(15.63) | 124 |
| abstract_inverted_index.(30.14) | 121 |
| abstract_inverted_index.(42.57) | 118 |
| abstract_inverted_index.acetone | 129 |
| abstract_inverted_index.alcohol | 132 |
| abstract_inverted_index.attempt | 1 |
| abstract_inverted_index.essence | 7 |
| abstract_inverted_index.ethanol | 120 |
| abstract_inverted_index.explore | 5 |
| abstract_inverted_index.length, | 66 |
| abstract_inverted_index.optimal | 150 |
| abstract_inverted_index.reflect | 143, 169 |
| abstract_inverted_index.results | 60 |
| abstract_inverted_index.species | 73 |
| abstract_inverted_index.stannic | 17, 40 |
| abstract_inverted_index.system. | 58 |
| abstract_inverted_index.testing | 57 |
| abstract_inverted_index.working | 175 |
| abstract_inverted_index.(10.32). | 130 |
| abstract_inverted_index.adsorbed | 138, 160 |
| abstract_inverted_index.analyzed | 43 |
| abstract_inverted_index.fluorine | 28, 167 |
| abstract_inverted_index.measured | 76 |
| abstract_inverted_index.methanol | 117 |
| abstract_inverted_index.obtained | 21 |
| abstract_inverted_index.produced | 165 |
| abstract_inverted_index.reactive | 14 |
| abstract_inverted_index.response | 110, 146 |
| abstract_inverted_index.sequence | 96 |
| abstract_inverted_index.activated | 30 |
| abstract_inverted_index.amorphous | 90 |
| abstract_inverted_index.combining | 23 |
| abstract_inverted_index.designing | 93 |
| abstract_inverted_index.determine | 78 |
| abstract_inverted_index.injection | 95 |
| abstract_inverted_index.position, | 69 |
| abstract_inverted_index.reduction | 25 |
| abstract_inverted_index.sequences | 111 |
| abstract_inverted_index.vacancies | 141 |
| abstract_inverted_index.1-propanol | 123 |
| abstract_inverted_index.2-propanol | 126 |
| abstract_inverted_index.functional | 67, 71 |
| abstract_inverted_index.increasing | 27 |
| abstract_inverted_index.molecules, | 107 |
| abstract_inverted_index.quaternary | 88 |
| abstract_inverted_index.gas-sensing | 37, 80 |
| abstract_inverted_index.microscopy, | 51 |
| abstract_inverted_index.performance | 38, 81 |
| abstract_inverted_index.selectivity | 10 |
| abstract_inverted_index.ultraviolet | 52 |
| abstract_inverted_index.diffraction, | 46 |
| abstract_inverted_index.irradiation. | 33 |
| abstract_inverted_index.precipitated | 86 |
| abstract_inverted_index.temperatures | 151, 176 |
| abstract_inverted_index.miscellaneous | 13 |
| abstract_inverted_index.spectroscopy, | 48 |
| abstract_inverted_index.microstructure | 35 |
| abstract_inverted_index.Zn–O–F–Sn | 89 |
| abstract_inverted_index.electrochemical | 56 |
| abstract_inverted_index.nanocrystalline | 84 |
| abstract_inverted_index.response/recovery | 171 |
| abstract_inverted_index.spectrophotometry, | 53 |
| abstract_inverted_index.SnO<sub>2–<i>x</i></sub>F<sub><i>y</i></sub> | 85, 114 |
| cited_by_percentile_year | |
| countries_distinct_count | 0 |
| institutions_distinct_count | 11 |
| citation_normalized_percentile.value | 0.70731343 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |