Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trial Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.3389/fimmu.2025.1519545
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.3389/fimmu.2025.1519545
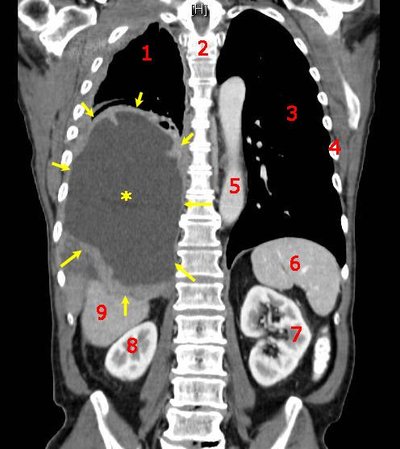
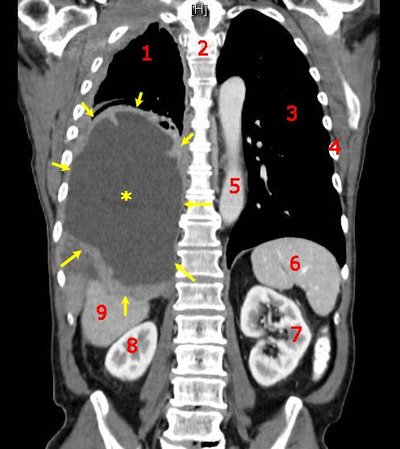
Background Gastric cancer (GC) is one of the most prevalent malignant tumors worldwide, often diagnosed at an advanced stage with a poor prognosis. Paclitaxel, nab-paclitaxel, and irinotecan, either as monotherapies or in combination with ramucirumab, are currently standard second-line treatments for GC. However, the efficacy of these therapies is limited, necessitating the development of new combination strategies to improve response rates. Immune checkpoint inhibitors (ICIs) have shown success in first-line treatment for advanced GC, leading to interest in immune rechallenge strategies for second-line treatment. Re-challenging patients with ICIs after progression on first-line treatment may restore immune responses and provide additional clinical benefit. Recently, cadonilimab (AK104), a bispecific antibody targeting PD-1 and CTLA-4, has demonstrated promising antitumor activity when combined with chemotherapy in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. However, the efficacy and safety of nab-paclitaxel combined with AK104 for the treatment of advanced GC remain unclear. Furthermore, identifying predictive biomarkers of efficacy is essential to developing personalized treatment strategies. This study aims to explore the safety and efficacy of nab-paclitaxel combined with AK104 as a second-line treatment for patients who have progressed after first-line chemoimmunotherapy, focusing on evaluating the therapeutic effect of ICIs rechallenge in gastric cancer. Methods This is a prospective, multicenter, open-label, single-arm Phase II clinical study. Eligible patients were histologically or cytologically diagnosed with unresectable recurrent or metastatic GC, failed first-line chemotherapy in combination with immune checkpoint inhibitor, aged between 18-75 years old, expected survival ≥3 months, and with a physical status of 0 or 1 in the Eastern Cooperative Cancer Group (ECOG). Enrolled patients will receive intravenous cadonilimab (AK104) 6 mg/kg on days 1, and 15, and intravenous nab-paclitaxel 100 mg/m 2 every four weeks on days 1, 8, and 15. The primary endpoints were objective response rate (ORR), and secondary endpoints were disease control rate (DCR), progression-free survival (PFS), and overall survival (OS). The exploratory objective was to identify biomarkers associated with efficacy, mechanism of action, and safety. A total of 59 participants were planned to be recruited using Simon’s two-stage design. The trial was initiated in June 2024 in China. Discussion This study is the first prospective trial to evaluate the combination of nab-paclitaxel and cadonilimab as second-line treatment after first-line chemoimmunotherapy failure. By investigating immune rechallenge, it aims to reactivate anti-tumor immune responses and improve clinical outcomes in GC patients. The exploration of predictive biomarkers, such as ctDNA, TMB, MSI, PD-L1 expression, TIL profiles, and gut microbiota, will help personalize treatment and identify patients most likely to benefit from immune rechallenge. This trial could provide valuable insights into overcoming immune resistance and contribute to developing a promising second-line therapeutic strategy for advanced GC. Clinical trial registration ClinicalTrials.Gov , identifier NCT06349967
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.3389/fimmu.2025.1519545
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdf
- OA Status
- gold
- Cited By
- 2
- References
- 60
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4407929111
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4407929111Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.3389/fimmu.2025.1519545Digital Object Identifier
- Title
-
Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trialWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-02-25Full publication date if available
- Authors
-
Jing Wei, Pengfei Zhang, Qiancheng Hu, Xiaolong Cheng, Chaoyong Shen, Zhixin Chen, Wen Zhuang, Yuan Yin, Bo Zhang, Hongfeng Gou, Kun Yang, Feng Bi, Ming LiuList of authors in order
- Landing page
-
https://doi.org/10.3389/fimmu.2025.1519545Publisher landing page
- PDF URL
-
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdfDirect OA link when available
- Concepts
-
Paclitaxel, Medicine, Cancer, Protocol (science), Oncology, Clinical trial, Internal medicine, Multicenter trial, Multicenter study, Randomized controlled trial, Pathology, Alternative medicineTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
2Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 2Per-year citation counts (last 5 years)
- References (count)
-
60Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4407929111 |
|---|---|
| doi | https://doi.org/10.3389/fimmu.2025.1519545 |
| ids.doi | https://doi.org/10.3389/fimmu.2025.1519545 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/40070819 |
| ids.openalex | https://openalex.org/W4407929111 |
| fwci | 9.77961587 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D006801 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Humans |
| mesh[1].qualifier_ui | Q000188 |
| mesh[1].descriptor_ui | D013274 |
| mesh[1].is_major_topic | True |
| mesh[1].qualifier_name | drug therapy |
| mesh[1].descriptor_name | Stomach Neoplasms |
| mesh[2].qualifier_ui | Q000276 |
| mesh[2].descriptor_ui | D013274 |
| mesh[2].is_major_topic | True |
| mesh[2].qualifier_name | immunology |
| mesh[2].descriptor_name | Stomach Neoplasms |
| mesh[3].qualifier_ui | Q000473 |
| mesh[3].descriptor_ui | D013274 |
| mesh[3].is_major_topic | True |
| mesh[3].qualifier_name | pathology |
| mesh[3].descriptor_name | Stomach Neoplasms |
| mesh[4].qualifier_ui | Q000008 |
| mesh[4].descriptor_ui | D017239 |
| mesh[4].is_major_topic | True |
| mesh[4].qualifier_name | administration & dosage |
| mesh[4].descriptor_name | Paclitaxel |
| mesh[5].qualifier_ui | Q000009 |
| mesh[5].descriptor_ui | D017239 |
| mesh[5].is_major_topic | True |
| mesh[5].qualifier_name | adverse effects |
| mesh[5].descriptor_name | Paclitaxel |
| mesh[6].qualifier_ui | Q000627 |
| mesh[6].descriptor_ui | D017239 |
| mesh[6].is_major_topic | True |
| mesh[6].qualifier_name | therapeutic use |
| mesh[6].descriptor_name | Paclitaxel |
| mesh[7].qualifier_ui | Q000008 |
| mesh[7].descriptor_ui | D000418 |
| mesh[7].is_major_topic | True |
| mesh[7].qualifier_name | administration & dosage |
| mesh[7].descriptor_name | Albumins |
| mesh[8].qualifier_ui | Q000627 |
| mesh[8].descriptor_ui | D000418 |
| mesh[8].is_major_topic | True |
| mesh[8].qualifier_name | therapeutic use |
| mesh[8].descriptor_name | Albumins |
| mesh[9].qualifier_ui | Q000627 |
| mesh[9].descriptor_ui | D000971 |
| mesh[9].is_major_topic | True |
| mesh[9].qualifier_name | therapeutic use |
| mesh[9].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[10].qualifier_ui | Q000009 |
| mesh[10].descriptor_ui | D000971 |
| mesh[10].is_major_topic | True |
| mesh[10].qualifier_name | adverse effects |
| mesh[10].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[11].qualifier_ui | |
| mesh[11].descriptor_ui | D011446 |
| mesh[11].is_major_topic | False |
| mesh[11].qualifier_name | |
| mesh[11].descriptor_name | Prospective Studies |
| mesh[12].qualifier_ui | |
| mesh[12].descriptor_ui | D008297 |
| mesh[12].is_major_topic | False |
| mesh[12].qualifier_name | |
| mesh[12].descriptor_name | Male |
| mesh[13].qualifier_ui | |
| mesh[13].descriptor_ui | D008875 |
| mesh[13].is_major_topic | False |
| mesh[13].qualifier_name | |
| mesh[13].descriptor_name | Middle Aged |
| mesh[14].qualifier_ui | |
| mesh[14].descriptor_ui | D005260 |
| mesh[14].is_major_topic | False |
| mesh[14].qualifier_name | |
| mesh[14].descriptor_name | Female |
| mesh[15].qualifier_ui | |
| mesh[15].descriptor_ui | D000328 |
| mesh[15].is_major_topic | False |
| mesh[15].qualifier_name | |
| mesh[15].descriptor_name | Adult |
| mesh[16].qualifier_ui | |
| mesh[16].descriptor_ui | D000368 |
| mesh[16].is_major_topic | False |
| mesh[16].qualifier_name | |
| mesh[16].descriptor_name | Aged |
| mesh[17].qualifier_ui | Q000008 |
| mesh[17].descriptor_ui | D061067 |
| mesh[17].is_major_topic | False |
| mesh[17].qualifier_name | administration & dosage |
| mesh[17].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[18].qualifier_ui | Q000627 |
| mesh[18].descriptor_ui | D061067 |
| mesh[18].is_major_topic | False |
| mesh[18].qualifier_name | therapeutic use |
| mesh[18].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[19].qualifier_ui | Q000009 |
| mesh[19].descriptor_ui | D061067 |
| mesh[19].is_major_topic | False |
| mesh[19].qualifier_name | adverse effects |
| mesh[19].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[20].qualifier_ui | Q000008 |
| mesh[20].descriptor_ui | D000082082 |
| mesh[20].is_major_topic | False |
| mesh[20].qualifier_name | administration & dosage |
| mesh[20].descriptor_name | Immune Checkpoint Inhibitors |
| mesh[21].qualifier_ui | Q000627 |
| mesh[21].descriptor_ui | D000082082 |
| mesh[21].is_major_topic | False |
| mesh[21].qualifier_name | therapeutic use |
| mesh[21].descriptor_name | Immune Checkpoint Inhibitors |
| mesh[22].qualifier_ui | Q000009 |
| mesh[22].descriptor_ui | D000082082 |
| mesh[22].is_major_topic | False |
| mesh[22].qualifier_name | adverse effects |
| mesh[22].descriptor_name | Immune Checkpoint Inhibitors |
| mesh[23].qualifier_ui | Q000008 |
| mesh[23].descriptor_ui | D018033 |
| mesh[23].is_major_topic | False |
| mesh[23].qualifier_name | administration & dosage |
| mesh[23].descriptor_name | Antibodies, Bispecific |
| mesh[24].qualifier_ui | Q000627 |
| mesh[24].descriptor_ui | D018033 |
| mesh[24].is_major_topic | False |
| mesh[24].qualifier_name | therapeutic use |
| mesh[24].descriptor_name | Antibodies, Bispecific |
| mesh[25].qualifier_ui | Q000009 |
| mesh[25].descriptor_ui | D018033 |
| mesh[25].is_major_topic | False |
| mesh[25].qualifier_name | adverse effects |
| mesh[25].descriptor_name | Antibodies, Bispecific |
| mesh[26].qualifier_ui | |
| mesh[26].descriptor_ui | D016896 |
| mesh[26].is_major_topic | False |
| mesh[26].qualifier_name | |
| mesh[26].descriptor_name | Treatment Outcome |
| mesh[27].qualifier_ui | |
| mesh[27].descriptor_ui | D006801 |
| mesh[27].is_major_topic | False |
| mesh[27].qualifier_name | |
| mesh[27].descriptor_name | Humans |
| mesh[28].qualifier_ui | Q000188 |
| mesh[28].descriptor_ui | D013274 |
| mesh[28].is_major_topic | True |
| mesh[28].qualifier_name | drug therapy |
| mesh[28].descriptor_name | Stomach Neoplasms |
| mesh[29].qualifier_ui | Q000276 |
| mesh[29].descriptor_ui | D013274 |
| mesh[29].is_major_topic | True |
| mesh[29].qualifier_name | immunology |
| mesh[29].descriptor_name | Stomach Neoplasms |
| mesh[30].qualifier_ui | Q000473 |
| mesh[30].descriptor_ui | D013274 |
| mesh[30].is_major_topic | True |
| mesh[30].qualifier_name | pathology |
| mesh[30].descriptor_name | Stomach Neoplasms |
| mesh[31].qualifier_ui | Q000008 |
| mesh[31].descriptor_ui | D017239 |
| mesh[31].is_major_topic | True |
| mesh[31].qualifier_name | administration & dosage |
| mesh[31].descriptor_name | Paclitaxel |
| mesh[32].qualifier_ui | Q000009 |
| mesh[32].descriptor_ui | D017239 |
| mesh[32].is_major_topic | True |
| mesh[32].qualifier_name | adverse effects |
| mesh[32].descriptor_name | Paclitaxel |
| mesh[33].qualifier_ui | Q000627 |
| mesh[33].descriptor_ui | D017239 |
| mesh[33].is_major_topic | True |
| mesh[33].qualifier_name | therapeutic use |
| mesh[33].descriptor_name | Paclitaxel |
| mesh[34].qualifier_ui | Q000008 |
| mesh[34].descriptor_ui | D000418 |
| mesh[34].is_major_topic | True |
| mesh[34].qualifier_name | administration & dosage |
| mesh[34].descriptor_name | Albumins |
| mesh[35].qualifier_ui | Q000627 |
| mesh[35].descriptor_ui | D000418 |
| mesh[35].is_major_topic | True |
| mesh[35].qualifier_name | therapeutic use |
| mesh[35].descriptor_name | Albumins |
| mesh[36].qualifier_ui | Q000627 |
| mesh[36].descriptor_ui | D000971 |
| mesh[36].is_major_topic | True |
| mesh[36].qualifier_name | therapeutic use |
| mesh[36].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[37].qualifier_ui | Q000009 |
| mesh[37].descriptor_ui | D000971 |
| mesh[37].is_major_topic | True |
| mesh[37].qualifier_name | adverse effects |
| mesh[37].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[38].qualifier_ui | |
| mesh[38].descriptor_ui | D011446 |
| mesh[38].is_major_topic | False |
| mesh[38].qualifier_name | |
| mesh[38].descriptor_name | Prospective Studies |
| mesh[39].qualifier_ui | |
| mesh[39].descriptor_ui | D008297 |
| mesh[39].is_major_topic | False |
| mesh[39].qualifier_name | |
| mesh[39].descriptor_name | Male |
| mesh[40].qualifier_ui | |
| mesh[40].descriptor_ui | D008875 |
| mesh[40].is_major_topic | False |
| mesh[40].qualifier_name | |
| mesh[40].descriptor_name | Middle Aged |
| mesh[41].qualifier_ui | |
| mesh[41].descriptor_ui | D005260 |
| mesh[41].is_major_topic | False |
| mesh[41].qualifier_name | |
| mesh[41].descriptor_name | Female |
| mesh[42].qualifier_ui | |
| mesh[42].descriptor_ui | D000328 |
| mesh[42].is_major_topic | False |
| mesh[42].qualifier_name | |
| mesh[42].descriptor_name | Adult |
| mesh[43].qualifier_ui | |
| mesh[43].descriptor_ui | D000368 |
| mesh[43].is_major_topic | False |
| mesh[43].qualifier_name | |
| mesh[43].descriptor_name | Aged |
| mesh[44].qualifier_ui | Q000008 |
| mesh[44].descriptor_ui | D061067 |
| mesh[44].is_major_topic | False |
| mesh[44].qualifier_name | administration & dosage |
| mesh[44].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[45].qualifier_ui | Q000627 |
| mesh[45].descriptor_ui | D061067 |
| mesh[45].is_major_topic | False |
| mesh[45].qualifier_name | therapeutic use |
| mesh[45].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[46].qualifier_ui | Q000009 |
| mesh[46].descriptor_ui | D061067 |
| mesh[46].is_major_topic | False |
| mesh[46].qualifier_name | adverse effects |
| mesh[46].descriptor_name | Antibodies, Monoclonal, Humanized |
| mesh[47].qualifier_ui | Q000008 |
| mesh[47].descriptor_ui | D000082082 |
| mesh[47].is_major_topic | False |
| mesh[47].qualifier_name | administration & dosage |
| mesh[47].descriptor_name | Immune Checkpoint Inhibitors |
| mesh[48].qualifier_ui | Q000627 |
| mesh[48].descriptor_ui | D000082082 |
| mesh[48].is_major_topic | False |
| mesh[48].qualifier_name | therapeutic use |
| mesh[48].descriptor_name | Immune Checkpoint Inhibitors |
| mesh[49].qualifier_ui | Q000009 |
| mesh[49].descriptor_ui | D000082082 |
| mesh[49].is_major_topic | False |
| mesh[49].qualifier_name | adverse effects |
| mesh[49].descriptor_name | Immune Checkpoint Inhibitors |
| type | article |
| title | Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trial |
| biblio.issue | |
| biblio.volume | 16 |
| biblio.last_page | 1519545 |
| biblio.first_page | 1519545 |
| topics[0].id | https://openalex.org/T10696 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9998999834060669 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2740 |
| topics[0].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[0].display_name | Gastric Cancer Management and Outcomes |
| topics[1].id | https://openalex.org/T10231 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9983999729156494 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2730 |
| topics[1].subfield.display_name | Oncology |
| topics[1].display_name | Pancreatic and Hepatic Oncology Research |
| topics[2].id | https://openalex.org/T13779 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9976999759674072 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2734 |
| topics[2].subfield.display_name | Pathology and Forensic Medicine |
| topics[2].display_name | Cancer Mechanisms and Therapy |
| is_xpac | False |
| apc_list.value | 2950 |
| apc_list.currency | USD |
| apc_list.value_usd | 2950 |
| apc_paid.value | 2950 |
| apc_paid.currency | USD |
| apc_paid.value_usd | 2950 |
| concepts[0].id | https://openalex.org/C2777292972 |
| concepts[0].level | 3 |
| concepts[0].score | 0.7762314081192017 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q423762 |
| concepts[0].display_name | Paclitaxel |
| concepts[1].id | https://openalex.org/C71924100 |
| concepts[1].level | 0 |
| concepts[1].score | 0.7422568202018738 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[1].display_name | Medicine |
| concepts[2].id | https://openalex.org/C121608353 |
| concepts[2].level | 2 |
| concepts[2].score | 0.5729383826255798 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[2].display_name | Cancer |
| concepts[3].id | https://openalex.org/C2780385302 |
| concepts[3].level | 3 |
| concepts[3].score | 0.47694841027259827 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q367158 |
| concepts[3].display_name | Protocol (science) |
| concepts[4].id | https://openalex.org/C143998085 |
| concepts[4].level | 1 |
| concepts[4].score | 0.4527658224105835 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q162555 |
| concepts[4].display_name | Oncology |
| concepts[5].id | https://openalex.org/C535046627 |
| concepts[5].level | 2 |
| concepts[5].score | 0.44411033391952515 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[5].display_name | Clinical trial |
| concepts[6].id | https://openalex.org/C126322002 |
| concepts[6].level | 1 |
| concepts[6].score | 0.4439808428287506 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[6].display_name | Internal medicine |
| concepts[7].id | https://openalex.org/C2775965419 |
| concepts[7].level | 4 |
| concepts[7].score | 0.42121422290802 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q6934595 |
| concepts[7].display_name | Multicenter trial |
| concepts[8].id | https://openalex.org/C2992435398 |
| concepts[8].level | 3 |
| concepts[8].score | 0.31086617708206177 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q6934595 |
| concepts[8].display_name | Multicenter study |
| concepts[9].id | https://openalex.org/C168563851 |
| concepts[9].level | 2 |
| concepts[9].score | 0.19364619255065918 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[9].display_name | Randomized controlled trial |
| concepts[10].id | https://openalex.org/C142724271 |
| concepts[10].level | 1 |
| concepts[10].score | 0.11651837825775146 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q7208 |
| concepts[10].display_name | Pathology |
| concepts[11].id | https://openalex.org/C204787440 |
| concepts[11].level | 2 |
| concepts[11].score | 0.0 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q188504 |
| concepts[11].display_name | Alternative medicine |
| keywords[0].id | https://openalex.org/keywords/paclitaxel |
| keywords[0].score | 0.7762314081192017 |
| keywords[0].display_name | Paclitaxel |
| keywords[1].id | https://openalex.org/keywords/medicine |
| keywords[1].score | 0.7422568202018738 |
| keywords[1].display_name | Medicine |
| keywords[2].id | https://openalex.org/keywords/cancer |
| keywords[2].score | 0.5729383826255798 |
| keywords[2].display_name | Cancer |
| keywords[3].id | https://openalex.org/keywords/protocol |
| keywords[3].score | 0.47694841027259827 |
| keywords[3].display_name | Protocol (science) |
| keywords[4].id | https://openalex.org/keywords/oncology |
| keywords[4].score | 0.4527658224105835 |
| keywords[4].display_name | Oncology |
| keywords[5].id | https://openalex.org/keywords/clinical-trial |
| keywords[5].score | 0.44411033391952515 |
| keywords[5].display_name | Clinical trial |
| keywords[6].id | https://openalex.org/keywords/internal-medicine |
| keywords[6].score | 0.4439808428287506 |
| keywords[6].display_name | Internal medicine |
| keywords[7].id | https://openalex.org/keywords/multicenter-trial |
| keywords[7].score | 0.42121422290802 |
| keywords[7].display_name | Multicenter trial |
| keywords[8].id | https://openalex.org/keywords/multicenter-study |
| keywords[8].score | 0.31086617708206177 |
| keywords[8].display_name | Multicenter study |
| keywords[9].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[9].score | 0.19364619255065918 |
| keywords[9].display_name | Randomized controlled trial |
| keywords[10].id | https://openalex.org/keywords/pathology |
| keywords[10].score | 0.11651837825775146 |
| keywords[10].display_name | Pathology |
| language | en |
| locations[0].id | doi:10.3389/fimmu.2025.1519545 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S2595292759 |
| locations[0].source.issn | 1664-3224 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 1664-3224 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Frontiers in Immunology |
| locations[0].source.host_organization | https://openalex.org/P4310320527 |
| locations[0].source.host_organization_name | Frontiers Media |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320527 |
| locations[0].source.host_organization_lineage_names | Frontiers Media |
| locations[0].license | cc-by |
| locations[0].pdf_url | https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Frontiers in Immunology |
| locations[0].landing_page_url | https://doi.org/10.3389/fimmu.2025.1519545 |
| locations[1].id | pmid:40070819 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Frontiers in immunology |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/40070819 |
| locations[2].id | pmh:oai:doaj.org/article:3d20cdaab3e34390a9f92315c18a47ac |
| locations[2].is_oa | False |
| locations[2].source.id | https://openalex.org/S4306401280 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[2].source.host_organization | |
| locations[2].source.host_organization_name | |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | article |
| locations[2].license_id | |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | Frontiers in Immunology, Vol 16 (2025) |
| locations[2].landing_page_url | https://doaj.org/article/3d20cdaab3e34390a9f92315c18a47ac |
| locations[3].id | pmh:oai:europepmc.org:10705715 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S4306400806 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | Europe PMC (PubMed Central) |
| locations[3].source.host_organization | https://openalex.org/I1303153112 |
| locations[3].source.host_organization_name | European Bioinformatics Institute |
| locations[3].source.host_organization_lineage | https://openalex.org/I1303153112 |
| locations[3].license | other-oa |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | Text |
| locations[3].license_id | https://openalex.org/licenses/other-oa |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | |
| locations[3].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/11893503 |
| indexed_in | crossref, doaj, pubmed |
| authorships[0].author.id | https://openalex.org/A5055587105 |
| authorships[0].author.orcid | https://orcid.org/0000-0002-2371-6111 |
| authorships[0].author.display_name | Jing Wei |
| authorships[0].countries | CN |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| authorships[0].affiliations[0].raw_affiliation_string | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[0].institutions[0].id | https://openalex.org/I4210118556 |
| authorships[0].institutions[0].ror | https://ror.org/029wq9x81 |
| authorships[0].institutions[0].type | healthcare |
| authorships[0].institutions[0].lineage | https://openalex.org/I4210118556 |
| authorships[0].institutions[0].country_code | CN |
| authorships[0].institutions[0].display_name | Sichuan Cancer Hospital |
| authorships[0].institutions[1].id | https://openalex.org/I24185976 |
| authorships[0].institutions[1].ror | https://ror.org/011ashp19 |
| authorships[0].institutions[1].type | education |
| authorships[0].institutions[1].lineage | https://openalex.org/I24185976 |
| authorships[0].institutions[1].country_code | CN |
| authorships[0].institutions[1].display_name | Sichuan University |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Jing Wei |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[1].author.id | https://openalex.org/A5100354135 |
| authorships[1].author.orcid | https://orcid.org/0000-0003-4437-8112 |
| authorships[1].author.display_name | Pengfei Zhang |
| authorships[1].countries | CN |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| authorships[1].affiliations[0].raw_affiliation_string | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[1].institutions[0].id | https://openalex.org/I4210118556 |
| authorships[1].institutions[0].ror | https://ror.org/029wq9x81 |
| authorships[1].institutions[0].type | healthcare |
| authorships[1].institutions[0].lineage | https://openalex.org/I4210118556 |
| authorships[1].institutions[0].country_code | CN |
| authorships[1].institutions[0].display_name | Sichuan Cancer Hospital |
| authorships[1].institutions[1].id | https://openalex.org/I24185976 |
| authorships[1].institutions[1].ror | https://ror.org/011ashp19 |
| authorships[1].institutions[1].type | education |
| authorships[1].institutions[1].lineage | https://openalex.org/I24185976 |
| authorships[1].institutions[1].country_code | CN |
| authorships[1].institutions[1].display_name | Sichuan University |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Pengfei Zhang |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[2].author.id | https://openalex.org/A5111177369 |
| authorships[2].author.orcid | |
| authorships[2].author.display_name | Qiancheng Hu |
| authorships[2].countries | CN |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| authorships[2].affiliations[0].raw_affiliation_string | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[2].institutions[0].id | https://openalex.org/I4210118556 |
| authorships[2].institutions[0].ror | https://ror.org/029wq9x81 |
| authorships[2].institutions[0].type | healthcare |
| authorships[2].institutions[0].lineage | https://openalex.org/I4210118556 |
| authorships[2].institutions[0].country_code | CN |
| authorships[2].institutions[0].display_name | Sichuan Cancer Hospital |
| authorships[2].institutions[1].id | https://openalex.org/I24185976 |
| authorships[2].institutions[1].ror | https://ror.org/011ashp19 |
| authorships[2].institutions[1].type | education |
| authorships[2].institutions[1].lineage | https://openalex.org/I24185976 |
| authorships[2].institutions[1].country_code | CN |
| authorships[2].institutions[1].display_name | Sichuan University |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Qiancheng Hu |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[3].author.id | https://openalex.org/A5109462654 |
| authorships[3].author.orcid | |
| authorships[3].author.display_name | Xiaolong Cheng |
| authorships[3].countries | CN |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[3].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[3].institutions[0].id | https://openalex.org/I24185976 |
| authorships[3].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[3].institutions[0].country_code | CN |
| authorships[3].institutions[0].display_name | Sichuan University |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Xiaolong Cheng |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[4].author.id | https://openalex.org/A5101547021 |
| authorships[4].author.orcid | https://orcid.org/0000-0002-8293-2746 |
| authorships[4].author.display_name | Chaoyong Shen |
| authorships[4].countries | CN |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[4].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[4].institutions[0].id | https://openalex.org/I24185976 |
| authorships[4].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[4].institutions[0].type | education |
| authorships[4].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[4].institutions[0].country_code | CN |
| authorships[4].institutions[0].display_name | Sichuan University |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Chaoyong Shen |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[5].author.id | https://openalex.org/A5100345059 |
| authorships[5].author.orcid | https://orcid.org/0000-0001-9222-9037 |
| authorships[5].author.display_name | Zhixin Chen |
| authorships[5].countries | CN |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[5].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[5].institutions[0].id | https://openalex.org/I24185976 |
| authorships[5].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[5].institutions[0].type | education |
| authorships[5].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[5].institutions[0].country_code | CN |
| authorships[5].institutions[0].display_name | Sichuan University |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Zhixin Chen |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[6].author.id | https://openalex.org/A5102407854 |
| authorships[6].author.orcid | |
| authorships[6].author.display_name | Wen Zhuang |
| authorships[6].countries | CN |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[6].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[6].institutions[0].id | https://openalex.org/I24185976 |
| authorships[6].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[6].institutions[0].type | education |
| authorships[6].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[6].institutions[0].country_code | CN |
| authorships[6].institutions[0].display_name | Sichuan University |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Wen Zhuang |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[7].author.id | https://openalex.org/A5100617690 |
| authorships[7].author.orcid | https://orcid.org/0000-0003-3359-4282 |
| authorships[7].author.display_name | Yuan Yin |
| authorships[7].countries | CN |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[7].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[7].institutions[0].id | https://openalex.org/I24185976 |
| authorships[7].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[7].institutions[0].type | education |
| authorships[7].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[7].institutions[0].country_code | CN |
| authorships[7].institutions[0].display_name | Sichuan University |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Yuan Yin |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[8].author.id | https://openalex.org/A5009980074 |
| authorships[8].author.orcid | https://orcid.org/0000-0002-6546-8443 |
| authorships[8].author.display_name | Bo Zhang |
| authorships[8].countries | CN |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[8].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[8].institutions[0].id | https://openalex.org/I24185976 |
| authorships[8].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[8].institutions[0].type | education |
| authorships[8].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[8].institutions[0].country_code | CN |
| authorships[8].institutions[0].display_name | Sichuan University |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Bo Zhang |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[9].author.id | https://openalex.org/A5006660603 |
| authorships[9].author.orcid | https://orcid.org/0000-0002-2071-6773 |
| authorships[9].author.display_name | Hongfeng Gou |
| authorships[9].countries | CN |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| authorships[9].affiliations[0].raw_affiliation_string | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[9].institutions[0].id | https://openalex.org/I4210118556 |
| authorships[9].institutions[0].ror | https://ror.org/029wq9x81 |
| authorships[9].institutions[0].type | healthcare |
| authorships[9].institutions[0].lineage | https://openalex.org/I4210118556 |
| authorships[9].institutions[0].country_code | CN |
| authorships[9].institutions[0].display_name | Sichuan Cancer Hospital |
| authorships[9].institutions[1].id | https://openalex.org/I24185976 |
| authorships[9].institutions[1].ror | https://ror.org/011ashp19 |
| authorships[9].institutions[1].type | education |
| authorships[9].institutions[1].lineage | https://openalex.org/I24185976 |
| authorships[9].institutions[1].country_code | CN |
| authorships[9].institutions[1].display_name | Sichuan University |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Hongfeng Gou |
| authorships[9].is_corresponding | False |
| authorships[9].raw_affiliation_strings | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[10].author.id | https://openalex.org/A5071799307 |
| authorships[10].author.orcid | https://orcid.org/0000-0002-8891-5736 |
| authorships[10].author.display_name | Kun Yang |
| authorships[10].countries | CN |
| authorships[10].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[10].affiliations[0].raw_affiliation_string | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[10].institutions[0].id | https://openalex.org/I24185976 |
| authorships[10].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[10].institutions[0].type | education |
| authorships[10].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[10].institutions[0].country_code | CN |
| authorships[10].institutions[0].display_name | Sichuan University |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Kun Yang |
| authorships[10].is_corresponding | False |
| authorships[10].raw_affiliation_strings | Department of General Surgery/Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[11].author.id | https://openalex.org/A5100567923 |
| authorships[11].author.orcid | |
| authorships[11].author.display_name | Feng Bi |
| authorships[11].countries | CN |
| authorships[11].affiliations[0].institution_ids | https://openalex.org/I24185976 |
| authorships[11].affiliations[0].raw_affiliation_string | Division of Abdominal Cancer, Department of Medical Oncology, Cancer Center and Laboratory of Molecular Targeted Therapy in Oncology, West China Hospital, Sichuan University, Chengdu, China |
| authorships[11].institutions[0].id | https://openalex.org/I24185976 |
| authorships[11].institutions[0].ror | https://ror.org/011ashp19 |
| authorships[11].institutions[0].type | education |
| authorships[11].institutions[0].lineage | https://openalex.org/I24185976 |
| authorships[11].institutions[0].country_code | CN |
| authorships[11].institutions[0].display_name | Sichuan University |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Feng Bi |
| authorships[11].is_corresponding | False |
| authorships[11].raw_affiliation_strings | Division of Abdominal Cancer, Department of Medical Oncology, Cancer Center and Laboratory of Molecular Targeted Therapy in Oncology, West China Hospital, Sichuan University, Chengdu, China |
| authorships[12].author.id | https://openalex.org/A5100347836 |
| authorships[12].author.orcid | https://orcid.org/0000-0003-3222-8715 |
| authorships[12].author.display_name | Ming Liu |
| authorships[12].countries | CN |
| authorships[12].affiliations[0].institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| authorships[12].affiliations[0].raw_affiliation_string | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| authorships[12].institutions[0].id | https://openalex.org/I4210118556 |
| authorships[12].institutions[0].ror | https://ror.org/029wq9x81 |
| authorships[12].institutions[0].type | healthcare |
| authorships[12].institutions[0].lineage | https://openalex.org/I4210118556 |
| authorships[12].institutions[0].country_code | CN |
| authorships[12].institutions[0].display_name | Sichuan Cancer Hospital |
| authorships[12].institutions[1].id | https://openalex.org/I24185976 |
| authorships[12].institutions[1].ror | https://ror.org/011ashp19 |
| authorships[12].institutions[1].type | education |
| authorships[12].institutions[1].lineage | https://openalex.org/I24185976 |
| authorships[12].institutions[1].country_code | CN |
| authorships[12].institutions[1].display_name | Sichuan University |
| authorships[12].author_position | last |
| authorships[12].raw_author_name | Ming Liu |
| authorships[12].is_corresponding | True |
| authorships[12].raw_affiliation_strings | Gastric Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China |
| has_content.pdf | True |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdf |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trial |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10696 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9998999834060669 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2740 |
| primary_topic.subfield.display_name | Pulmonary and Respiratory Medicine |
| primary_topic.display_name | Gastric Cancer Management and Outcomes |
| related_works | https://openalex.org/W2497183490, https://openalex.org/W2799885860, https://openalex.org/W2586534962, https://openalex.org/W1993529741, https://openalex.org/W3165335631, https://openalex.org/W2416650349, https://openalex.org/W2037238905, https://openalex.org/W2460365608, https://openalex.org/W3085985127, https://openalex.org/W2414075721 |
| cited_by_count | 2 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 2 |
| locations_count | 4 |
| best_oa_location.id | doi:10.3389/fimmu.2025.1519545 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S2595292759 |
| best_oa_location.source.issn | 1664-3224 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 1664-3224 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Frontiers in Immunology |
| best_oa_location.source.host_organization | https://openalex.org/P4310320527 |
| best_oa_location.source.host_organization_name | Frontiers Media |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320527 |
| best_oa_location.source.host_organization_lineage_names | Frontiers Media |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Frontiers in Immunology |
| best_oa_location.landing_page_url | https://doi.org/10.3389/fimmu.2025.1519545 |
| primary_location.id | doi:10.3389/fimmu.2025.1519545 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S2595292759 |
| primary_location.source.issn | 1664-3224 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 1664-3224 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Frontiers in Immunology |
| primary_location.source.host_organization | https://openalex.org/P4310320527 |
| primary_location.source.host_organization_name | Frontiers Media |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320527 |
| primary_location.source.host_organization_lineage_names | Frontiers Media |
| primary_location.license | cc-by |
| primary_location.pdf_url | https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1519545/pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Frontiers in Immunology |
| primary_location.landing_page_url | https://doi.org/10.3389/fimmu.2025.1519545 |
| publication_date | 2025-02-25 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W4390946922, https://openalex.org/W4308463946, https://openalex.org/W4401899170, https://openalex.org/W4285490947, https://openalex.org/W4392091243, https://openalex.org/W4389326921, https://openalex.org/W4387849849, https://openalex.org/W4387809571, https://openalex.org/W4398209645, https://openalex.org/W4401381330, https://openalex.org/W4403119976, https://openalex.org/W4400577186, https://openalex.org/W4396765271, https://openalex.org/W4393931466, https://openalex.org/W4382049038, https://openalex.org/W4399257026, https://openalex.org/W3205914652, https://openalex.org/W4401729464, https://openalex.org/W4402524285, https://openalex.org/W4390734340, https://openalex.org/W3121059150, https://openalex.org/W4292485486, https://openalex.org/W4402951343, https://openalex.org/W4403502708, https://openalex.org/W4403243250, https://openalex.org/W4401996447, https://openalex.org/W4377088207, https://openalex.org/W4403228155, https://openalex.org/W4403000621, https://openalex.org/W4402859830, https://openalex.org/W2577864197, https://openalex.org/W4323294881, https://openalex.org/W4392167095, https://openalex.org/W4387709297, https://openalex.org/W4387246689, https://openalex.org/W4396640272, https://openalex.org/W4313553529, https://openalex.org/W4386817778, https://openalex.org/W3171517418, https://openalex.org/W4206384216, https://openalex.org/W4391329310, https://openalex.org/W4399762369, https://openalex.org/W4391577415, https://openalex.org/W4386600575, https://openalex.org/W4402518045, https://openalex.org/W4401698627, https://openalex.org/W4386211830, https://openalex.org/W4402396948, https://openalex.org/W4321613266, https://openalex.org/W3109005927, https://openalex.org/W3090469545, https://openalex.org/W3217126890, https://openalex.org/W4402552706, https://openalex.org/W3007099934, https://openalex.org/W4387951276, https://openalex.org/W4401452195, https://openalex.org/W4401173872, https://openalex.org/W4401237747, https://openalex.org/W4394987899, https://openalex.org/W4387996822 |
| referenced_works_count | 60 |
| abstract_inverted_index., | 445 |
| abstract_inverted_index.0 | 247 |
| abstract_inverted_index.1 | 249 |
| abstract_inverted_index.2 | 276 |
| abstract_inverted_index.6 | 264 |
| abstract_inverted_index.A | 324 |
| abstract_inverted_index.a | 20, 105, 175, 201, 243, 433 |
| abstract_inverted_index.1, | 268, 282 |
| abstract_inverted_index.59 | 327 |
| abstract_inverted_index.8, | 283 |
| abstract_inverted_index.By | 370 |
| abstract_inverted_index.GC | 144, 386 |
| abstract_inverted_index.II | 207 |
| abstract_inverted_index.an | 16 |
| abstract_inverted_index.as | 28, 174, 363, 394 |
| abstract_inverted_index.at | 15 |
| abstract_inverted_index.be | 332 |
| abstract_inverted_index.in | 31, 68, 77, 121, 195, 226, 250, 342, 345, 385 |
| abstract_inverted_index.is | 4, 48, 153, 200, 350 |
| abstract_inverted_index.it | 374 |
| abstract_inverted_index.of | 6, 45, 53, 134, 142, 151, 169, 192, 246, 320, 326, 359, 390 |
| abstract_inverted_index.on | 90, 187, 266, 280 |
| abstract_inverted_index.or | 30, 214, 220, 248 |
| abstract_inverted_index.to | 57, 75, 155, 163, 313, 331, 355, 376, 414, 431 |
| abstract_inverted_index.100 | 274 |
| abstract_inverted_index.15, | 270 |
| abstract_inverted_index.15. | 285 |
| abstract_inverted_index.GC, | 73, 222 |
| abstract_inverted_index.GC. | 41, 440 |
| abstract_inverted_index.TIL | 400 |
| abstract_inverted_index.The | 286, 309, 338, 388 |
| abstract_inverted_index.and | 25, 97, 110, 124, 132, 167, 241, 269, 271, 284, 294, 305, 322, 361, 381, 402, 409, 429 |
| abstract_inverted_index.are | 35 |
| abstract_inverted_index.for | 40, 71, 81, 139, 178, 438 |
| abstract_inverted_index.gut | 403 |
| abstract_inverted_index.has | 112 |
| abstract_inverted_index.may | 93 |
| abstract_inverted_index.new | 54 |
| abstract_inverted_index.one | 5 |
| abstract_inverted_index.the | 7, 43, 51, 130, 140, 165, 189, 251, 351, 357 |
| abstract_inverted_index.was | 312, 340 |
| abstract_inverted_index.who | 180 |
| abstract_inverted_index.(GC) | 3 |
| abstract_inverted_index.2024 | 344 |
| abstract_inverted_index.ICIs | 87, 193 |
| abstract_inverted_index.June | 343 |
| abstract_inverted_index.MSI, | 397 |
| abstract_inverted_index.PD-1 | 109 |
| abstract_inverted_index.TMB, | 396 |
| abstract_inverted_index.This | 160, 199, 348, 419 |
| abstract_inverted_index.aged | 232 |
| abstract_inverted_index.aims | 162, 375 |
| abstract_inverted_index.days | 267, 281 |
| abstract_inverted_index.four | 278 |
| abstract_inverted_index.from | 416 |
| abstract_inverted_index.have | 65, 181 |
| abstract_inverted_index.help | 406 |
| abstract_inverted_index.into | 425 |
| abstract_inverted_index.mg/m | 275 |
| abstract_inverted_index.most | 8, 412 |
| abstract_inverted_index.old, | 236 |
| abstract_inverted_index.poor | 21 |
| abstract_inverted_index.rate | 292, 300 |
| abstract_inverted_index.such | 393 |
| abstract_inverted_index.were | 212, 289, 297, 329 |
| abstract_inverted_index.when | 117 |
| abstract_inverted_index.will | 259, 405 |
| abstract_inverted_index.with | 19, 33, 86, 119, 137, 172, 217, 228, 242, 317 |
| abstract_inverted_index.≥3 | 239 |
| abstract_inverted_index.(GEJ) | 127 |
| abstract_inverted_index.(OS). | 308 |
| abstract_inverted_index.18-75 | 234 |
| abstract_inverted_index.AK104 | 138, 173 |
| abstract_inverted_index.Group | 255 |
| abstract_inverted_index.PD-L1 | 398 |
| abstract_inverted_index.Phase | 206 |
| abstract_inverted_index.after | 88, 183, 366 |
| abstract_inverted_index.could | 421 |
| abstract_inverted_index.every | 277 |
| abstract_inverted_index.first | 352 |
| abstract_inverted_index.mg/kg | 265 |
| abstract_inverted_index.often | 13 |
| abstract_inverted_index.shown | 66 |
| abstract_inverted_index.stage | 18 |
| abstract_inverted_index.study | 161, 349 |
| abstract_inverted_index.these | 46 |
| abstract_inverted_index.total | 325 |
| abstract_inverted_index.trial | 339, 354, 420, 442 |
| abstract_inverted_index.using | 334 |
| abstract_inverted_index.weeks | 279 |
| abstract_inverted_index.years | 235 |
| abstract_inverted_index.(DCR), | 301 |
| abstract_inverted_index.(ICIs) | 64 |
| abstract_inverted_index.(ORR), | 293 |
| abstract_inverted_index.(PFS), | 304 |
| abstract_inverted_index.Cancer | 254 |
| abstract_inverted_index.China. | 346 |
| abstract_inverted_index.Immune | 61 |
| abstract_inverted_index.cancer | 2 |
| abstract_inverted_index.ctDNA, | 395 |
| abstract_inverted_index.effect | 191 |
| abstract_inverted_index.either | 27 |
| abstract_inverted_index.failed | 223 |
| abstract_inverted_index.immune | 78, 95, 229, 372, 379, 417, 427 |
| abstract_inverted_index.likely | 413 |
| abstract_inverted_index.rates. | 60 |
| abstract_inverted_index.remain | 145 |
| abstract_inverted_index.safety | 133, 166 |
| abstract_inverted_index.status | 245 |
| abstract_inverted_index.study. | 209 |
| abstract_inverted_index.tumors | 11 |
| abstract_inverted_index.(AK104) | 263 |
| abstract_inverted_index.(ECOG). | 256 |
| abstract_inverted_index.CTLA-4, | 111 |
| abstract_inverted_index.Eastern | 252 |
| abstract_inverted_index.Gastric | 1 |
| abstract_inverted_index.Methods | 198 |
| abstract_inverted_index.action, | 321 |
| abstract_inverted_index.benefit | 415 |
| abstract_inverted_index.between | 233 |
| abstract_inverted_index.cancer. | 197 |
| abstract_inverted_index.control | 299 |
| abstract_inverted_index.design. | 337 |
| abstract_inverted_index.disease | 298 |
| abstract_inverted_index.explore | 164 |
| abstract_inverted_index.gastric | 123, 196 |
| abstract_inverted_index.improve | 58, 382 |
| abstract_inverted_index.leading | 74 |
| abstract_inverted_index.months, | 240 |
| abstract_inverted_index.overall | 306 |
| abstract_inverted_index.planned | 330 |
| abstract_inverted_index.primary | 287 |
| abstract_inverted_index.provide | 98, 422 |
| abstract_inverted_index.receive | 260 |
| abstract_inverted_index.restore | 94 |
| abstract_inverted_index.safety. | 323 |
| abstract_inverted_index.success | 67 |
| abstract_inverted_index.(AK104), | 104 |
| abstract_inverted_index.Clinical | 441 |
| abstract_inverted_index.Eligible | 210 |
| abstract_inverted_index.Enrolled | 257 |
| abstract_inverted_index.However, | 42, 129 |
| abstract_inverted_index.activity | 116 |
| abstract_inverted_index.advanced | 17, 72, 122, 143, 439 |
| abstract_inverted_index.antibody | 107 |
| abstract_inverted_index.benefit. | 101 |
| abstract_inverted_index.clinical | 100, 208, 383 |
| abstract_inverted_index.combined | 118, 136, 171 |
| abstract_inverted_index.efficacy | 44, 131, 152, 168 |
| abstract_inverted_index.evaluate | 356 |
| abstract_inverted_index.expected | 237 |
| abstract_inverted_index.failure. | 369 |
| abstract_inverted_index.focusing | 186 |
| abstract_inverted_index.identify | 314, 410 |
| abstract_inverted_index.insights | 424 |
| abstract_inverted_index.interest | 76 |
| abstract_inverted_index.junction | 126 |
| abstract_inverted_index.limited, | 49 |
| abstract_inverted_index.outcomes | 384 |
| abstract_inverted_index.patients | 85, 179, 211, 258, 411 |
| abstract_inverted_index.physical | 244 |
| abstract_inverted_index.response | 59, 291 |
| abstract_inverted_index.standard | 37 |
| abstract_inverted_index.strategy | 437 |
| abstract_inverted_index.survival | 238, 303, 307 |
| abstract_inverted_index.unclear. | 146 |
| abstract_inverted_index.valuable | 423 |
| abstract_inverted_index.Recently, | 102 |
| abstract_inverted_index.Simon’s | 335 |
| abstract_inverted_index.antitumor | 115 |
| abstract_inverted_index.currently | 36 |
| abstract_inverted_index.diagnosed | 14, 216 |
| abstract_inverted_index.efficacy, | 318 |
| abstract_inverted_index.endpoints | 288, 296 |
| abstract_inverted_index.essential | 154 |
| abstract_inverted_index.initiated | 341 |
| abstract_inverted_index.malignant | 10 |
| abstract_inverted_index.mechanism | 319 |
| abstract_inverted_index.objective | 290, 311 |
| abstract_inverted_index.patients. | 387 |
| abstract_inverted_index.prevalent | 9 |
| abstract_inverted_index.profiles, | 401 |
| abstract_inverted_index.promising | 114, 434 |
| abstract_inverted_index.recruited | 333 |
| abstract_inverted_index.recurrent | 219 |
| abstract_inverted_index.responses | 96, 380 |
| abstract_inverted_index.secondary | 295 |
| abstract_inverted_index.targeting | 108 |
| abstract_inverted_index.therapies | 47 |
| abstract_inverted_index.treatment | 70, 92, 141, 158, 177, 365, 408 |
| abstract_inverted_index.two-stage | 336 |
| abstract_inverted_index.Background | 0 |
| abstract_inverted_index.Discussion | 347 |
| abstract_inverted_index.additional | 99 |
| abstract_inverted_index.anti-tumor | 378 |
| abstract_inverted_index.associated | 316 |
| abstract_inverted_index.biomarkers | 150, 315 |
| abstract_inverted_index.bispecific | 106 |
| abstract_inverted_index.checkpoint | 62, 230 |
| abstract_inverted_index.contribute | 430 |
| abstract_inverted_index.developing | 156, 432 |
| abstract_inverted_index.evaluating | 188 |
| abstract_inverted_index.first-line | 69, 91, 184, 224, 367 |
| abstract_inverted_index.identifier | 446 |
| abstract_inverted_index.inhibitor, | 231 |
| abstract_inverted_index.inhibitors | 63 |
| abstract_inverted_index.metastatic | 221 |
| abstract_inverted_index.overcoming | 426 |
| abstract_inverted_index.predictive | 149, 391 |
| abstract_inverted_index.prognosis. | 22 |
| abstract_inverted_index.progressed | 182 |
| abstract_inverted_index.reactivate | 377 |
| abstract_inverted_index.resistance | 428 |
| abstract_inverted_index.single-arm | 205 |
| abstract_inverted_index.strategies | 56, 80 |
| abstract_inverted_index.treatment. | 83 |
| abstract_inverted_index.treatments | 39 |
| abstract_inverted_index.worldwide, | 12 |
| abstract_inverted_index.Cooperative | 253 |
| abstract_inverted_index.NCT06349967 | 447 |
| abstract_inverted_index.Paclitaxel, | 23 |
| abstract_inverted_index.biomarkers, | 392 |
| abstract_inverted_index.cadonilimab | 103, 262, 362 |
| abstract_inverted_index.combination | 32, 55, 227, 358 |
| abstract_inverted_index.development | 52 |
| abstract_inverted_index.exploration | 389 |
| abstract_inverted_index.exploratory | 310 |
| abstract_inverted_index.expression, | 399 |
| abstract_inverted_index.identifying | 148 |
| abstract_inverted_index.intravenous | 261, 272 |
| abstract_inverted_index.irinotecan, | 26 |
| abstract_inverted_index.microbiota, | 404 |
| abstract_inverted_index.open-label, | 204 |
| abstract_inverted_index.personalize | 407 |
| abstract_inverted_index.progression | 89 |
| abstract_inverted_index.prospective | 353 |
| abstract_inverted_index.rechallenge | 79, 194 |
| abstract_inverted_index.second-line | 38, 82, 176, 364, 435 |
| abstract_inverted_index.strategies. | 159 |
| abstract_inverted_index.therapeutic | 190, 436 |
| abstract_inverted_index.Furthermore, | 147 |
| abstract_inverted_index.chemotherapy | 120, 225 |
| abstract_inverted_index.demonstrated | 113 |
| abstract_inverted_index.multicenter, | 203 |
| abstract_inverted_index.participants | 328 |
| abstract_inverted_index.personalized | 157 |
| abstract_inverted_index.prospective, | 202 |
| abstract_inverted_index.ramucirumab, | 34 |
| abstract_inverted_index.rechallenge, | 373 |
| abstract_inverted_index.rechallenge. | 418 |
| abstract_inverted_index.registration | 443 |
| abstract_inverted_index.unresectable | 218 |
| abstract_inverted_index.cytologically | 215 |
| abstract_inverted_index.investigating | 371 |
| abstract_inverted_index.monotherapies | 29 |
| abstract_inverted_index.necessitating | 50 |
| abstract_inverted_index.Re-challenging | 84 |
| abstract_inverted_index.histologically | 213 |
| abstract_inverted_index.nab-paclitaxel | 135, 170, 273, 360 |
| abstract_inverted_index.adenocarcinoma. | 128 |
| abstract_inverted_index.nab-paclitaxel, | 24 |
| abstract_inverted_index.gastroesophageal | 125 |
| abstract_inverted_index.progression-free | 302 |
| abstract_inverted_index.ClinicalTrials.Gov | 444 |
| abstract_inverted_index.chemoimmunotherapy | 368 |
| abstract_inverted_index.chemoimmunotherapy, | 185 |
| cited_by_percentile_year.max | 97 |
| cited_by_percentile_year.min | 95 |
| corresponding_author_ids | https://openalex.org/A5100347836 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 13 |
| corresponding_institution_ids | https://openalex.org/I24185976, https://openalex.org/I4210118556 |
| citation_normalized_percentile.value | 0.93232822 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | True |