Network polymers containing disulfide bond prepared by addition reactions of multi-functional epoxies, aziridine, or isocyanates with dicarboxylic acids or diols: structure, mechanical properties, and reductive degradation Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.21203/rs.3.rs-7874491/v1
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.21203/rs.3.rs-7874491/v1
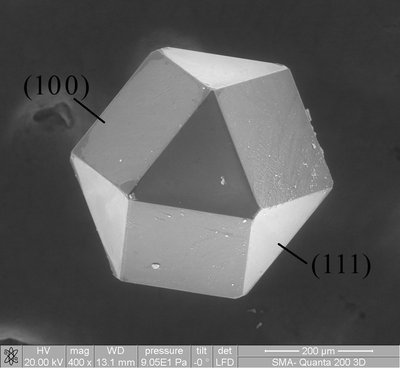
Network polymers containing disulfide bonds were synthesized through ring-opening addition reactions of multi-functional epoxides or a tri-aziridine with dithiodicarboxylic acids. Reactions of tri- or tetra-functional epoxide, namely tris(4-hydroxyphenyl) methane triglycidyl ether (TME) and tetraphenylolethane glycidyl ether (TPE), with dithiodicarboxylic acids of varying methylene chain length, dithiodiglycolic acid (DTGA), 3,3'-dithiodipropionic acid (DTPA), or 4,4'-dithiodibutyric acid (DTBA), in the presence of tetrabutylammonium bromide in dimethyl sulfoxide (DMSO) at 85 ºC afforded the corresponding gels. Gels prepared from DTBA exhibited an enhanced Young’s modulus, attributed to the high extent of reaction conversion. These gels underwent reductive degradation upon immersion in a DMSO solution of dithiothreitol (DTT), while subsequent heating of the resulting solutions regenerated the gel structures through oxidation mediated by DMSO. Ring-opening addition reaction of tri-aziridine, 2,2-bishydroxymethylbutanol-tris[3-(1-aziridinyl)propionate] (3AZ) with dithiodicarboxylic acids in methanol successfully yielded porous polymers via polymerization induced phase separation (PIPS). The resulting materials exhibited porous morphologies composed of interconnected particles with diameters ranging from approximately 1 to 4 µm. The particle size increased with the alkyl length of the dithiodicarboxylic acid, concomitant with a reduction in the Young’s modulus of the porous polymers. In addition, the reaction of tri-functional isocyanate, 1,3,5-Tris[6-(isocyanate)hexyl]-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (3I) with bis(2-hydroxyethyl) disulfide (HEDS) or DTGA in DMSO produced the corresponding gels. These gels were susceptible to reductive degradation in DMSO solutions of DTT, and notably, the 3I-HEDS gel was degradable under electrochemical reduction. The reaction of 3I with DTGA in tetrahydrofuran (THF) afforded porous polymers exhibiting co-continuous monolithic morphologies consisting of interconnected particles. The morphology was tunable by varying concentration of triethylamine employed as a catalyst. The 3I-DTGA porous polymer likewise underwent reductive degradation upon immersion in THF solution of DTT.
Related Topics
- Type
- article
- Landing Page
- https://doi.org/10.21203/rs.3.rs-7874491/v1
- https://www.researchsquare.com/article/rs-7874491/latest.pdf
- OA Status
- gold
- References
- 29
- OpenAlex ID
- https://openalex.org/W7105677643
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W7105677643Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.21203/rs.3.rs-7874491/v1Digital Object Identifier
- Title
-
Network polymers containing disulfide bond prepared by addition reactions of multi-functional epoxies, aziridine, or isocyanates with dicarboxylic acids or diols: structure, mechanical properties, and reductive degradationWork title
- Type
-
articleOpenAlex work type
- Publication year
-
2025Year of publication
- Publication date
-
2025-11-14Full publication date if available
- Authors
-
Naofumi Naga, Kazumasa Moriyama, Kazuma Hasegawa, Toshiki Tajima, Tamaki NakanoList of authors in order
- Landing page
-
https://doi.org/10.21203/rs.3.rs-7874491/v1Publisher landing page
- PDF URL
-
https://www.researchsquare.com/article/rs-7874491/latest.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://www.researchsquare.com/article/rs-7874491/latest.pdfDirect OA link when available
- Concepts
-
Chemistry, Polymer chemistry, Polymer, Ether, Tetrahydrofuran, Polymerization, Alkyl, Sulfoxide, Dimethyl sulfoxide, Bromide, Dicarboxylic acid, Methanol, Redox, Acetic acid, Organic chemistry, Cationic polymerization, Addition reaction, Covalent bond, Peroxide, Silsesquioxane, Cross-link, Aryl, Alkoxy group, Allyl bromide, Materials scienceTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- References (count)
-
29Number of works referenced by this work
Full payload
| id | https://openalex.org/W7105677643 |
|---|---|
| doi | https://doi.org/10.21203/rs.3.rs-7874491/v1 |
| ids.doi | https://doi.org/10.21203/rs.3.rs-7874491/v1 |
| ids.openalex | https://openalex.org/W7105677643 |
| fwci | 0.0 |
| type | article |
| title | Network polymers containing disulfide bond prepared by addition reactions of multi-functional epoxies, aziridine, or isocyanates with dicarboxylic acids or diols: structure, mechanical properties, and reductive degradation |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | |
| biblio.first_page | |
| topics[0].id | https://openalex.org/T11593 |
| topics[0].field.id | https://openalex.org/fields/15 |
| topics[0].field.display_name | Chemical Engineering |
| topics[0].score | 0.3938225507736206 |
| topics[0].domain.id | https://openalex.org/domains/3 |
| topics[0].domain.display_name | Physical Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/1508 |
| topics[0].subfield.display_name | Process Chemistry and Technology |
| topics[0].display_name | Carbon dioxide utilization in catalysis |
| topics[1].id | https://openalex.org/T12038 |
| topics[1].field.id | https://openalex.org/fields/25 |
| topics[1].field.display_name | Materials Science |
| topics[1].score | 0.3559240996837616 |
| topics[1].domain.id | https://openalex.org/domains/3 |
| topics[1].domain.display_name | Physical Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2505 |
| topics[1].subfield.display_name | Materials Chemistry |
| topics[1].display_name | Covalent Organic Framework Applications |
| topics[2].id | https://openalex.org/T12570 |
| topics[2].field.id | https://openalex.org/fields/16 |
| topics[2].field.display_name | Chemistry |
| topics[2].score | 0.048928532749414444 |
| topics[2].domain.id | https://openalex.org/domains/3 |
| topics[2].domain.display_name | Physical Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/1605 |
| topics[2].subfield.display_name | Organic Chemistry |
| topics[2].display_name | Photopolymerization techniques and applications |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C185592680 |
| concepts[0].level | 0 |
| concepts[0].score | 0.6382436156272888 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q2329 |
| concepts[0].display_name | Chemistry |
| concepts[1].id | https://openalex.org/C188027245 |
| concepts[1].level | 1 |
| concepts[1].score | 0.6123597025871277 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q750446 |
| concepts[1].display_name | Polymer chemistry |
| concepts[2].id | https://openalex.org/C521977710 |
| concepts[2].level | 2 |
| concepts[2].score | 0.5543394684791565 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q81163 |
| concepts[2].display_name | Polymer |
| concepts[3].id | https://openalex.org/C2780407432 |
| concepts[3].level | 2 |
| concepts[3].score | 0.5542446374893188 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q103230 |
| concepts[3].display_name | Ether |
| concepts[4].id | https://openalex.org/C2776701986 |
| concepts[4].level | 3 |
| concepts[4].score | 0.5461754202842712 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q278332 |
| concepts[4].display_name | Tetrahydrofuran |
| concepts[5].id | https://openalex.org/C44228677 |
| concepts[5].level | 3 |
| concepts[5].score | 0.5254030823707581 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q181898 |
| concepts[5].display_name | Polymerization |
| concepts[6].id | https://openalex.org/C2780263894 |
| concepts[6].level | 2 |
| concepts[6].score | 0.441948264837265 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q335268 |
| concepts[6].display_name | Alkyl |
| concepts[7].id | https://openalex.org/C2778448320 |
| concepts[7].level | 2 |
| concepts[7].score | 0.4374825358390808 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q408395 |
| concepts[7].display_name | Sulfoxide |
| concepts[8].id | https://openalex.org/C2778452553 |
| concepts[8].level | 2 |
| concepts[8].score | 0.43681958317756653 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q407927 |
| concepts[8].display_name | Dimethyl sulfoxide |
| concepts[9].id | https://openalex.org/C2776300020 |
| concepts[9].level | 2 |
| concepts[9].score | 0.41132986545562744 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q422423 |
| concepts[9].display_name | Bromide |
| concepts[10].id | https://openalex.org/C2779146789 |
| concepts[10].level | 2 |
| concepts[10].score | 0.4045126438140869 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q422050 |
| concepts[10].display_name | Dicarboxylic acid |
| concepts[11].id | https://openalex.org/C2779607525 |
| concepts[11].level | 2 |
| concepts[11].score | 0.37124285101890564 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q14982 |
| concepts[11].display_name | Methanol |
| concepts[12].id | https://openalex.org/C55904794 |
| concepts[12].level | 2 |
| concepts[12].score | 0.36855655908584595 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q82682 |
| concepts[12].display_name | Redox |
| concepts[13].id | https://openalex.org/C2776673659 |
| concepts[13].level | 2 |
| concepts[13].score | 0.3602229654788971 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q47512 |
| concepts[13].display_name | Acetic acid |
| concepts[14].id | https://openalex.org/C178790620 |
| concepts[14].level | 1 |
| concepts[14].score | 0.3504802882671356 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q11351 |
| concepts[14].display_name | Organic chemistry |
| concepts[15].id | https://openalex.org/C183882617 |
| concepts[15].level | 2 |
| concepts[15].score | 0.31700167059898376 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q3046749 |
| concepts[15].display_name | Cationic polymerization |
| concepts[16].id | https://openalex.org/C92832382 |
| concepts[16].level | 3 |
| concepts[16].score | 0.3044365644454956 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q212745 |
| concepts[16].display_name | Addition reaction |
| concepts[17].id | https://openalex.org/C180577832 |
| concepts[17].level | 2 |
| concepts[17].score | 0.3016411066055298 |
| concepts[17].wikidata | https://www.wikidata.org/wiki/Q127920 |
| concepts[17].display_name | Covalent bond |
| concepts[18].id | https://openalex.org/C2781048764 |
| concepts[18].level | 2 |
| concepts[18].score | 0.2859032452106476 |
| concepts[18].wikidata | https://www.wikidata.org/wiki/Q107429 |
| concepts[18].display_name | Peroxide |
| concepts[19].id | https://openalex.org/C2779063765 |
| concepts[19].level | 3 |
| concepts[19].score | 0.2697773575782776 |
| concepts[19].wikidata | https://www.wikidata.org/wiki/Q4354961 |
| concepts[19].display_name | Silsesquioxane |
| concepts[20].id | https://openalex.org/C142708767 |
| concepts[20].level | 3 |
| concepts[20].score | 0.2647424638271332 |
| concepts[20].wikidata | https://www.wikidata.org/wiki/Q898597 |
| concepts[20].display_name | Cross-link |
| concepts[21].id | https://openalex.org/C2781076698 |
| concepts[21].level | 3 |
| concepts[21].score | 0.2621530592441559 |
| concepts[21].wikidata | https://www.wikidata.org/wiki/Q718074 |
| concepts[21].display_name | Aryl |
| concepts[22].id | https://openalex.org/C90150868 |
| concepts[22].level | 3 |
| concepts[22].score | 0.25999781489372253 |
| concepts[22].wikidata | https://www.wikidata.org/wiki/Q39513 |
| concepts[22].display_name | Alkoxy group |
| concepts[23].id | https://openalex.org/C2778675482 |
| concepts[23].level | 2 |
| concepts[23].score | 0.25983303785324097 |
| concepts[23].wikidata | https://www.wikidata.org/wiki/Q223062 |
| concepts[23].display_name | Allyl bromide |
| concepts[24].id | https://openalex.org/C192562407 |
| concepts[24].level | 0 |
| concepts[24].score | 0.2521466314792633 |
| concepts[24].wikidata | https://www.wikidata.org/wiki/Q228736 |
| concepts[24].display_name | Materials science |
| keywords[0].id | https://openalex.org/keywords/polymer |
| keywords[0].score | 0.5543394684791565 |
| keywords[0].display_name | Polymer |
| keywords[1].id | https://openalex.org/keywords/ether |
| keywords[1].score | 0.5542446374893188 |
| keywords[1].display_name | Ether |
| keywords[2].id | https://openalex.org/keywords/tetrahydrofuran |
| keywords[2].score | 0.5461754202842712 |
| keywords[2].display_name | Tetrahydrofuran |
| keywords[3].id | https://openalex.org/keywords/polymerization |
| keywords[3].score | 0.5254030823707581 |
| keywords[3].display_name | Polymerization |
| keywords[4].id | https://openalex.org/keywords/alkyl |
| keywords[4].score | 0.441948264837265 |
| keywords[4].display_name | Alkyl |
| keywords[5].id | https://openalex.org/keywords/sulfoxide |
| keywords[5].score | 0.4374825358390808 |
| keywords[5].display_name | Sulfoxide |
| keywords[6].id | https://openalex.org/keywords/dimethyl-sulfoxide |
| keywords[6].score | 0.43681958317756653 |
| keywords[6].display_name | Dimethyl sulfoxide |
| keywords[7].id | https://openalex.org/keywords/bromide |
| keywords[7].score | 0.41132986545562744 |
| keywords[7].display_name | Bromide |
| keywords[8].id | https://openalex.org/keywords/dicarboxylic-acid |
| keywords[8].score | 0.4045126438140869 |
| keywords[8].display_name | Dicarboxylic acid |
| language | |
| locations[0].id | doi:10.21203/rs.3.rs-7874491/v1 |
| locations[0].is_oa | True |
| locations[0].source | |
| locations[0].license | cc-by |
| locations[0].pdf_url | https://www.researchsquare.com/article/rs-7874491/latest.pdf |
| locations[0].version | acceptedVersion |
| locations[0].raw_type | posted-content |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | False |
| locations[0].raw_source_name | |
| locations[0].landing_page_url | https://doi.org/10.21203/rs.3.rs-7874491/v1 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A2205078754 |
| authorships[0].author.orcid | https://orcid.org/0000-0003-0695-5011 |
| authorships[0].author.display_name | Naofumi Naga |
| authorships[0].countries | JP |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I171481255 |
| authorships[0].affiliations[0].raw_affiliation_string | Shibaura Institute of Technology: Shibaura Kogyo Daigaku |
| authorships[0].institutions[0].id | https://openalex.org/I171481255 |
| authorships[0].institutions[0].ror | https://ror.org/https://ror.org/020wjcq07 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I171481255 |
| authorships[0].institutions[0].country_code | JP |
| authorships[0].institutions[0].display_name | Shibaura Institute of Technology |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Naofumi Naga |
| authorships[0].is_corresponding | True |
| authorships[0].raw_affiliation_strings | Shibaura Institute of Technology: Shibaura Kogyo Daigaku |
| authorships[1].author.id | https://openalex.org/A2746271823 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Kazumasa Moriyama |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Kazumasa Moriyama |
| authorships[1].is_corresponding | False |
| authorships[2].author.id | https://openalex.org/A2396112481 |
| authorships[2].author.orcid | |
| authorships[2].author.display_name | Kazuma Hasegawa |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Kazuma Hasegawa |
| authorships[2].is_corresponding | False |
| authorships[3].author.id | https://openalex.org/A2472172445 |
| authorships[3].author.orcid | |
| authorships[3].author.display_name | Toshiki Tajima |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Toshiki Tajima |
| authorships[3].is_corresponding | False |
| authorships[4].author.id | https://openalex.org/A2112808026 |
| authorships[4].author.orcid | https://orcid.org/0000-0002-7843-4146 |
| authorships[4].author.display_name | Tamaki Nakano |
| authorships[4].author_position | last |
| authorships[4].raw_author_name | Tamaki Nakano |
| authorships[4].is_corresponding | False |
| has_content.pdf | True |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://www.researchsquare.com/article/rs-7874491/latest.pdf |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-11-14T00:00:00 |
| display_name | Network polymers containing disulfide bond prepared by addition reactions of multi-functional epoxies, aziridine, or isocyanates with dicarboxylic acids or diols: structure, mechanical properties, and reductive degradation |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-15T23:13:30.683059 |
| primary_topic.id | https://openalex.org/T11593 |
| primary_topic.field.id | https://openalex.org/fields/15 |
| primary_topic.field.display_name | Chemical Engineering |
| primary_topic.score | 0.3938225507736206 |
| primary_topic.domain.id | https://openalex.org/domains/3 |
| primary_topic.domain.display_name | Physical Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/1508 |
| primary_topic.subfield.display_name | Process Chemistry and Technology |
| primary_topic.display_name | Carbon dioxide utilization in catalysis |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | doi:10.21203/rs.3.rs-7874491/v1 |
| best_oa_location.is_oa | True |
| best_oa_location.source | |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | https://www.researchsquare.com/article/rs-7874491/latest.pdf |
| best_oa_location.version | acceptedVersion |
| best_oa_location.raw_type | posted-content |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | |
| best_oa_location.landing_page_url | https://doi.org/10.21203/rs.3.rs-7874491/v1 |
| primary_location.id | doi:10.21203/rs.3.rs-7874491/v1 |
| primary_location.is_oa | True |
| primary_location.source | |
| primary_location.license | cc-by |
| primary_location.pdf_url | https://www.researchsquare.com/article/rs-7874491/latest.pdf |
| primary_location.version | acceptedVersion |
| primary_location.raw_type | posted-content |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | False |
| primary_location.raw_source_name | |
| primary_location.landing_page_url | https://doi.org/10.21203/rs.3.rs-7874491/v1 |
| publication_date | 2025-11-14 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W2143843435, https://openalex.org/W4403548190, https://openalex.org/W4393931433, https://openalex.org/W4318046487, https://openalex.org/W3198616637, https://openalex.org/W4387456785, https://openalex.org/W3172955754, https://openalex.org/W2047074031, https://openalex.org/W4296642220, https://openalex.org/W2945483507, https://openalex.org/W2900482885, https://openalex.org/W2762738574, https://openalex.org/W3164313088, https://openalex.org/W4396749862, https://openalex.org/W3122024542, https://openalex.org/W3019223604, https://openalex.org/W3173716801, https://openalex.org/W4309739395, https://openalex.org/W4385860583, https://openalex.org/W4304080358, https://openalex.org/W4312250347, https://openalex.org/W2111276299, https://openalex.org/W2997004046, https://openalex.org/W2289292636, https://openalex.org/W2985760152, https://openalex.org/W3209881608, https://openalex.org/W4311185822, https://openalex.org/W2748015696, https://openalex.org/W2092726852 |
| referenced_works_count | 29 |
| abstract_inverted_index.1 | 157 |
| abstract_inverted_index.4 | 159 |
| abstract_inverted_index.a | 16, 98, 175, 259 |
| abstract_inverted_index.3I | 231 |
| abstract_inverted_index.85 | 67 |
| abstract_inverted_index.In | 185 |
| abstract_inverted_index.an | 78 |
| abstract_inverted_index.as | 258 |
| abstract_inverted_index.at | 66 |
| abstract_inverted_index.by | 118, 252 |
| abstract_inverted_index.in | 56, 62, 97, 130, 177, 200, 213, 234, 271 |
| abstract_inverted_index.of | 12, 22, 41, 59, 87, 101, 107, 123, 149, 169, 181, 189, 216, 230, 245, 255, 274 |
| abstract_inverted_index.or | 15, 24, 52, 198 |
| abstract_inverted_index.to | 83, 158, 210 |
| abstract_inverted_index.THF | 272 |
| abstract_inverted_index.The | 142, 161, 228, 248, 261 |
| abstract_inverted_index.and | 33, 218 |
| abstract_inverted_index.gel | 113, 222 |
| abstract_inverted_index.the | 57, 70, 84, 108, 112, 166, 170, 178, 182, 187, 203, 220 |
| abstract_inverted_index.via | 136 |
| abstract_inverted_index.was | 223, 250 |
| abstract_inverted_index.ºC | 68 |
| abstract_inverted_index.(3I) | 193 |
| abstract_inverted_index.DMSO | 99, 201, 214 |
| abstract_inverted_index.DTBA | 76 |
| abstract_inverted_index.DTGA | 199, 233 |
| abstract_inverted_index.DTT, | 217 |
| abstract_inverted_index.DTT. | 275 |
| abstract_inverted_index.Gels | 73 |
| abstract_inverted_index.acid | 47, 50, 54 |
| abstract_inverted_index.from | 75, 155 |
| abstract_inverted_index.gels | 91, 207 |
| abstract_inverted_index.high | 85 |
| abstract_inverted_index.size | 163 |
| abstract_inverted_index.tri- | 23 |
| abstract_inverted_index.upon | 95, 269 |
| abstract_inverted_index.were | 6, 208 |
| abstract_inverted_index.with | 18, 38, 127, 152, 165, 174, 194, 232 |
| abstract_inverted_index.µm. | 160 |
| abstract_inverted_index.(3AZ) | 126 |
| abstract_inverted_index.(THF) | 236 |
| abstract_inverted_index.(TME) | 32 |
| abstract_inverted_index.DMSO. | 119 |
| abstract_inverted_index.These | 90, 206 |
| abstract_inverted_index.acid, | 172 |
| abstract_inverted_index.acids | 40, 129 |
| abstract_inverted_index.alkyl | 167 |
| abstract_inverted_index.bonds | 5 |
| abstract_inverted_index.chain | 44 |
| abstract_inverted_index.ether | 31, 36 |
| abstract_inverted_index.gels. | 72, 205 |
| abstract_inverted_index.phase | 139 |
| abstract_inverted_index.under | 225 |
| abstract_inverted_index.while | 104 |
| abstract_inverted_index.(DMSO) | 65 |
| abstract_inverted_index.(DTT), | 103 |
| abstract_inverted_index.(HEDS) | 197 |
| abstract_inverted_index.(TPE), | 37 |
| abstract_inverted_index.acids. | 20 |
| abstract_inverted_index.extent | 86 |
| abstract_inverted_index.length | 168 |
| abstract_inverted_index.namely | 27 |
| abstract_inverted_index.porous | 134, 146, 183, 238, 263 |
| abstract_inverted_index.(DTBA), | 55 |
| abstract_inverted_index.(DTGA), | 48 |
| abstract_inverted_index.(DTPA), | 51 |
| abstract_inverted_index.(PIPS). | 141 |
| abstract_inverted_index.3I-DTGA | 262 |
| abstract_inverted_index.3I-HEDS | 221 |
| abstract_inverted_index.Network | 1 |
| abstract_inverted_index.bromide | 61 |
| abstract_inverted_index.heating | 106 |
| abstract_inverted_index.induced | 138 |
| abstract_inverted_index.length, | 45 |
| abstract_inverted_index.methane | 29 |
| abstract_inverted_index.modulus | 180 |
| abstract_inverted_index.polymer | 264 |
| abstract_inverted_index.ranging | 154 |
| abstract_inverted_index.through | 8, 115 |
| abstract_inverted_index.tunable | 251 |
| abstract_inverted_index.varying | 42, 253 |
| abstract_inverted_index.yielded | 133 |
| abstract_inverted_index.addition | 10, 121 |
| abstract_inverted_index.afforded | 69, 237 |
| abstract_inverted_index.composed | 148 |
| abstract_inverted_index.dimethyl | 63 |
| abstract_inverted_index.employed | 257 |
| abstract_inverted_index.enhanced | 79 |
| abstract_inverted_index.epoxide, | 26 |
| abstract_inverted_index.epoxides | 14 |
| abstract_inverted_index.glycidyl | 35 |
| abstract_inverted_index.likewise | 265 |
| abstract_inverted_index.mediated | 117 |
| abstract_inverted_index.methanol | 131 |
| abstract_inverted_index.modulus, | 81 |
| abstract_inverted_index.notably, | 219 |
| abstract_inverted_index.particle | 162 |
| abstract_inverted_index.polymers | 2, 135, 239 |
| abstract_inverted_index.prepared | 74 |
| abstract_inverted_index.presence | 58 |
| abstract_inverted_index.produced | 202 |
| abstract_inverted_index.reaction | 88, 122, 188, 229 |
| abstract_inverted_index.solution | 100, 273 |
| abstract_inverted_index.Reactions | 21 |
| abstract_inverted_index.Young’s | 80, 179 |
| abstract_inverted_index.addition, | 186 |
| abstract_inverted_index.catalyst. | 260 |
| abstract_inverted_index.diameters | 153 |
| abstract_inverted_index.disulfide | 4, 196 |
| abstract_inverted_index.exhibited | 77, 145 |
| abstract_inverted_index.immersion | 96, 270 |
| abstract_inverted_index.increased | 164 |
| abstract_inverted_index.materials | 144 |
| abstract_inverted_index.methylene | 43 |
| abstract_inverted_index.oxidation | 116 |
| abstract_inverted_index.particles | 151 |
| abstract_inverted_index.polymers. | 184 |
| abstract_inverted_index.reactions | 11 |
| abstract_inverted_index.reduction | 176 |
| abstract_inverted_index.reductive | 93, 211, 267 |
| abstract_inverted_index.resulting | 109, 143 |
| abstract_inverted_index.solutions | 110, 215 |
| abstract_inverted_index.sulfoxide | 64 |
| abstract_inverted_index.underwent | 92, 266 |
| abstract_inverted_index.attributed | 82 |
| abstract_inverted_index.consisting | 244 |
| abstract_inverted_index.containing | 3 |
| abstract_inverted_index.degradable | 224 |
| abstract_inverted_index.exhibiting | 240 |
| abstract_inverted_index.monolithic | 242 |
| abstract_inverted_index.morphology | 249 |
| abstract_inverted_index.particles. | 247 |
| abstract_inverted_index.reduction. | 227 |
| abstract_inverted_index.separation | 140 |
| abstract_inverted_index.structures | 114 |
| abstract_inverted_index.subsequent | 105 |
| abstract_inverted_index.concomitant | 173 |
| abstract_inverted_index.conversion. | 89 |
| abstract_inverted_index.degradation | 94, 212, 268 |
| abstract_inverted_index.isocyanate, | 191 |
| abstract_inverted_index.regenerated | 111 |
| abstract_inverted_index.susceptible | 209 |
| abstract_inverted_index.synthesized | 7 |
| abstract_inverted_index.triglycidyl | 30 |
| abstract_inverted_index.Ring-opening | 120 |
| abstract_inverted_index.morphologies | 147, 243 |
| abstract_inverted_index.ring-opening | 9 |
| abstract_inverted_index.successfully | 132 |
| abstract_inverted_index.approximately | 156 |
| abstract_inverted_index.co-continuous | 241 |
| abstract_inverted_index.concentration | 254 |
| abstract_inverted_index.corresponding | 71, 204 |
| abstract_inverted_index.tri-aziridine | 17 |
| abstract_inverted_index.triethylamine | 256 |
| abstract_inverted_index.dithiothreitol | 102 |
| abstract_inverted_index.interconnected | 150, 246 |
| abstract_inverted_index.polymerization | 137 |
| abstract_inverted_index.tri-aziridine, | 124 |
| abstract_inverted_index.tri-functional | 190 |
| abstract_inverted_index.electrochemical | 226 |
| abstract_inverted_index.tetrahydrofuran | 235 |
| abstract_inverted_index.dithiodiglycolic | 46 |
| abstract_inverted_index.multi-functional | 13 |
| abstract_inverted_index.tetra-functional | 25 |
| abstract_inverted_index.dithiodicarboxylic | 19, 39, 128, 171 |
| abstract_inverted_index.tetrabutylammonium | 60 |
| abstract_inverted_index.bis(2-hydroxyethyl) | 195 |
| abstract_inverted_index.tetraphenylolethane | 34 |
| abstract_inverted_index.4,4'-dithiodibutyric | 53 |
| abstract_inverted_index.tris(4-hydroxyphenyl) | 28 |
| abstract_inverted_index.3,3'-dithiodipropionic | 49 |
| abstract_inverted_index.<title>Abstract</title> | 0 |
| abstract_inverted_index.2,2-bishydroxymethylbutanol-tris[3-(1-aziridinyl)propionate] | 125 |
| abstract_inverted_index.1,3,5-Tris[6-(isocyanate)hexyl]-1,3,5-triazine-2,4,6(1H,3H,5H)-trione | 192 |
| cited_by_percentile_year | |
| countries_distinct_count | 1 |
| institutions_distinct_count | 5 |
| citation_normalized_percentile.value | 0.6571683 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |