Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1038/s41591-025-03746-z
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1038/s41591-025-03746-z
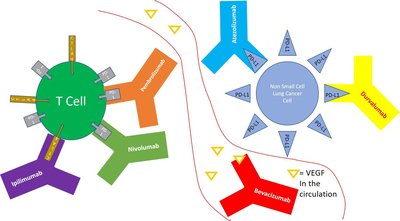
In the phase II NeoCOAST-2 platform study, 202 patients with untreated, resectable stage IIA–IIIB non-small-cell lung cancer (NSCLC) were randomized to receive neoadjuvant durvalumab plus platinum-doublet chemotherapy with oleclumab, a CD73 inhibitor (Arm 1), or with monalizumab, a NKG2A inhibitor (Arm 2), or neoadjuvant durvalumab plus single-agent platinum chemotherapy with the TROP-2 antibody–drug conjugate (ADC) datopotamab deruxtecan (Arm 4), followed by surgical resection and adjuvant durvalumab with oleclumab or monalizumab (Arms 1 and 2) or durvalumab alone (Arm 4). Primary endpoints were pathological complete response (pCR) rate and safety; secondary endpoints included feasibility of surgery and major pathological response (mPR) rate. In the modified intention-to-treat population ( n = 198; Arm 1, n = 74; Arm 2, n = 70; Arm 4, n = 54), pCR rates were 20.3% (15/74; 95% CI, 11.8–31.2), 25.7% (18/70; 95% CI, 16.0–37.6) and 35.2% (19/54; 95% CI, 22.7–49.4), and mPR rates were 41.9% (31/74; 95% CI, 30.5–53.9), 50.0% (35/70; 95% CI, 37.8–62.2) and 63.0% (34/54; 95% CI, 48.7–75.7) in arms 1, 2, and 4, respectively. In the safety population, 69/74 (93.2%), 66/71 (93.0%), and 51/54 (94.4%) patients underwent surgery, respectively. Overall, grade ≥3 treatment-related adverse events occurred in 27/74 (36.5%), 29/71 (40.8%) and 11/54 (20.4%) patients, respectively. In NeoCOAST-2, the first neoadjuvant trial examining an ADC plus chemo-immunotherapy in resectable NSCLC, pCR rates were highest in the datopotamab-deruxtecan-containing arm, warranting further investigation in larger trials of ADCs and checkpoint inhibition in the neoadjuvant setting. ClinicalTrials.gov identifier: NCT05061550 .
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1038/s41591-025-03746-z
- OA Status
- hybrid
- Cited By
- 8
- References
- 22
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4410915155
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4410915155Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1038/s41591-025-03746-zDigital Object Identifier
- Title
-
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trialWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-05-31Full publication date if available
- Authors
-
Tina Cascone, Laura Bonanno, Florian Guisier, Amelia Insa, Moïshe Liberman, Olivier Bylicki, Lorenzo Livi, Thomas Egenod, R. Corre, Dong‐Wan Kim, Rosario García-Campelo, Mariano Provencio, Byoung Yong Shim, Giulio Metro, Jaafar Bennouna, Agata A. Bielska, Alula R. Yohannes, Yun He, Adam Dowson, Gozde Kar, Lara McGrath, Rakesh Kumar, Italia Grenga, Jonathan Spicer, Patrick M. FordeList of authors in order
- Landing page
-
https://doi.org/10.1038/s41591-025-03746-zPublisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
hybridOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.1038/s41591-025-03746-zDirect OA link when available
- Concepts
-
Durvalumab, Medicine, Perioperative, Chemotherapy, Lung cancer, Population, Clinical endpoint, Internal medicine, Adverse effect, Surgery, Phases of clinical research, Urology, Gastroenterology, Cancer, Clinical trial, Immunotherapy, Nivolumab, Environmental healthTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
8Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 8Per-year citation counts (last 5 years)
- References (count)
-
22Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4410915155 |
|---|---|
| doi | https://doi.org/10.1038/s41591-025-03746-z |
| ids.doi | https://doi.org/10.1038/s41591-025-03746-z |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/40450142 |
| ids.openalex | https://openalex.org/W4410915155 |
| fwci | 39.11846347 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D006801 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Humans |
| mesh[1].qualifier_ui | Q000188 |
| mesh[1].descriptor_ui | D002289 |
| mesh[1].is_major_topic | True |
| mesh[1].qualifier_name | drug therapy |
| mesh[1].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[2].qualifier_ui | Q000601 |
| mesh[2].descriptor_ui | D002289 |
| mesh[2].is_major_topic | True |
| mesh[2].qualifier_name | surgery |
| mesh[2].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[3].qualifier_ui | Q000473 |
| mesh[3].descriptor_ui | D002289 |
| mesh[3].is_major_topic | True |
| mesh[3].qualifier_name | pathology |
| mesh[3].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[4].qualifier_ui | |
| mesh[4].descriptor_ui | D005260 |
| mesh[4].is_major_topic | False |
| mesh[4].qualifier_name | |
| mesh[4].descriptor_name | Female |
| mesh[5].qualifier_ui | |
| mesh[5].descriptor_ui | D008297 |
| mesh[5].is_major_topic | False |
| mesh[5].qualifier_name | |
| mesh[5].descriptor_name | Male |
| mesh[6].qualifier_ui | |
| mesh[6].descriptor_ui | D008875 |
| mesh[6].is_major_topic | False |
| mesh[6].qualifier_name | |
| mesh[6].descriptor_name | Middle Aged |
| mesh[7].qualifier_ui | Q000188 |
| mesh[7].descriptor_ui | D008175 |
| mesh[7].is_major_topic | True |
| mesh[7].qualifier_name | drug therapy |
| mesh[7].descriptor_name | Lung Neoplasms |
| mesh[8].qualifier_ui | Q000601 |
| mesh[8].descriptor_ui | D008175 |
| mesh[8].is_major_topic | True |
| mesh[8].qualifier_name | surgery |
| mesh[8].descriptor_name | Lung Neoplasms |
| mesh[9].qualifier_ui | Q000473 |
| mesh[9].descriptor_ui | D008175 |
| mesh[9].is_major_topic | True |
| mesh[9].qualifier_name | pathology |
| mesh[9].descriptor_name | Lung Neoplasms |
| mesh[10].qualifier_ui | |
| mesh[10].descriptor_ui | D000368 |
| mesh[10].is_major_topic | False |
| mesh[10].qualifier_name | |
| mesh[10].descriptor_name | Aged |
| mesh[11].qualifier_ui | Q000008 |
| mesh[11].descriptor_ui | D000911 |
| mesh[11].is_major_topic | True |
| mesh[11].qualifier_name | administration & dosage |
| mesh[11].descriptor_name | Antibodies, Monoclonal |
| mesh[12].qualifier_ui | Q000627 |
| mesh[12].descriptor_ui | D000911 |
| mesh[12].is_major_topic | True |
| mesh[12].qualifier_name | therapeutic use |
| mesh[12].descriptor_name | Antibodies, Monoclonal |
| mesh[13].qualifier_ui | Q000009 |
| mesh[13].descriptor_ui | D000911 |
| mesh[13].is_major_topic | True |
| mesh[13].qualifier_name | adverse effects |
| mesh[13].descriptor_name | Antibodies, Monoclonal |
| mesh[14].qualifier_ui | Q000627 |
| mesh[14].descriptor_ui | D000971 |
| mesh[14].is_major_topic | True |
| mesh[14].qualifier_name | therapeutic use |
| mesh[14].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[15].qualifier_ui | Q000009 |
| mesh[15].descriptor_ui | D000971 |
| mesh[15].is_major_topic | True |
| mesh[15].qualifier_name | adverse effects |
| mesh[15].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[16].qualifier_ui | |
| mesh[16].descriptor_ui | D000328 |
| mesh[16].is_major_topic | False |
| mesh[16].qualifier_name | |
| mesh[16].descriptor_name | Adult |
| mesh[17].qualifier_ui | |
| mesh[17].descriptor_ui | D020360 |
| mesh[17].is_major_topic | False |
| mesh[17].qualifier_name | |
| mesh[17].descriptor_name | Neoadjuvant Therapy |
| mesh[18].qualifier_ui | |
| mesh[18].descriptor_ui | D016896 |
| mesh[18].is_major_topic | False |
| mesh[18].qualifier_name | |
| mesh[18].descriptor_name | Treatment Outcome |
| mesh[19].qualifier_ui | |
| mesh[19].descriptor_ui | D006801 |
| mesh[19].is_major_topic | False |
| mesh[19].qualifier_name | |
| mesh[19].descriptor_name | Humans |
| mesh[20].qualifier_ui | Q000188 |
| mesh[20].descriptor_ui | D002289 |
| mesh[20].is_major_topic | True |
| mesh[20].qualifier_name | drug therapy |
| mesh[20].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[21].qualifier_ui | Q000601 |
| mesh[21].descriptor_ui | D002289 |
| mesh[21].is_major_topic | True |
| mesh[21].qualifier_name | surgery |
| mesh[21].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[22].qualifier_ui | Q000473 |
| mesh[22].descriptor_ui | D002289 |
| mesh[22].is_major_topic | True |
| mesh[22].qualifier_name | pathology |
| mesh[22].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[23].qualifier_ui | |
| mesh[23].descriptor_ui | D005260 |
| mesh[23].is_major_topic | False |
| mesh[23].qualifier_name | |
| mesh[23].descriptor_name | Female |
| mesh[24].qualifier_ui | |
| mesh[24].descriptor_ui | D008297 |
| mesh[24].is_major_topic | False |
| mesh[24].qualifier_name | |
| mesh[24].descriptor_name | Male |
| mesh[25].qualifier_ui | |
| mesh[25].descriptor_ui | D008875 |
| mesh[25].is_major_topic | False |
| mesh[25].qualifier_name | |
| mesh[25].descriptor_name | Middle Aged |
| mesh[26].qualifier_ui | Q000188 |
| mesh[26].descriptor_ui | D008175 |
| mesh[26].is_major_topic | True |
| mesh[26].qualifier_name | drug therapy |
| mesh[26].descriptor_name | Lung Neoplasms |
| mesh[27].qualifier_ui | Q000601 |
| mesh[27].descriptor_ui | D008175 |
| mesh[27].is_major_topic | True |
| mesh[27].qualifier_name | surgery |
| mesh[27].descriptor_name | Lung Neoplasms |
| mesh[28].qualifier_ui | Q000473 |
| mesh[28].descriptor_ui | D008175 |
| mesh[28].is_major_topic | True |
| mesh[28].qualifier_name | pathology |
| mesh[28].descriptor_name | Lung Neoplasms |
| mesh[29].qualifier_ui | |
| mesh[29].descriptor_ui | D000368 |
| mesh[29].is_major_topic | False |
| mesh[29].qualifier_name | |
| mesh[29].descriptor_name | Aged |
| mesh[30].qualifier_ui | Q000008 |
| mesh[30].descriptor_ui | D000911 |
| mesh[30].is_major_topic | True |
| mesh[30].qualifier_name | administration & dosage |
| mesh[30].descriptor_name | Antibodies, Monoclonal |
| mesh[31].qualifier_ui | Q000627 |
| mesh[31].descriptor_ui | D000911 |
| mesh[31].is_major_topic | True |
| mesh[31].qualifier_name | therapeutic use |
| mesh[31].descriptor_name | Antibodies, Monoclonal |
| mesh[32].qualifier_ui | Q000009 |
| mesh[32].descriptor_ui | D000911 |
| mesh[32].is_major_topic | True |
| mesh[32].qualifier_name | adverse effects |
| mesh[32].descriptor_name | Antibodies, Monoclonal |
| mesh[33].qualifier_ui | Q000627 |
| mesh[33].descriptor_ui | D000971 |
| mesh[33].is_major_topic | True |
| mesh[33].qualifier_name | therapeutic use |
| mesh[33].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[34].qualifier_ui | Q000009 |
| mesh[34].descriptor_ui | D000971 |
| mesh[34].is_major_topic | True |
| mesh[34].qualifier_name | adverse effects |
| mesh[34].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[35].qualifier_ui | |
| mesh[35].descriptor_ui | D000328 |
| mesh[35].is_major_topic | False |
| mesh[35].qualifier_name | |
| mesh[35].descriptor_name | Adult |
| mesh[36].qualifier_ui | |
| mesh[36].descriptor_ui | D020360 |
| mesh[36].is_major_topic | False |
| mesh[36].qualifier_name | |
| mesh[36].descriptor_name | Neoadjuvant Therapy |
| mesh[37].qualifier_ui | |
| mesh[37].descriptor_ui | D016896 |
| mesh[37].is_major_topic | False |
| mesh[37].qualifier_name | |
| mesh[37].descriptor_name | Treatment Outcome |
| mesh[38].qualifier_ui | |
| mesh[38].descriptor_ui | D006801 |
| mesh[38].is_major_topic | False |
| mesh[38].qualifier_name | |
| mesh[38].descriptor_name | Humans |
| mesh[39].qualifier_ui | Q000188 |
| mesh[39].descriptor_ui | D002289 |
| mesh[39].is_major_topic | True |
| mesh[39].qualifier_name | drug therapy |
| mesh[39].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[40].qualifier_ui | Q000601 |
| mesh[40].descriptor_ui | D002289 |
| mesh[40].is_major_topic | True |
| mesh[40].qualifier_name | surgery |
| mesh[40].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[41].qualifier_ui | Q000473 |
| mesh[41].descriptor_ui | D002289 |
| mesh[41].is_major_topic | True |
| mesh[41].qualifier_name | pathology |
| mesh[41].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[42].qualifier_ui | |
| mesh[42].descriptor_ui | D005260 |
| mesh[42].is_major_topic | False |
| mesh[42].qualifier_name | |
| mesh[42].descriptor_name | Female |
| mesh[43].qualifier_ui | |
| mesh[43].descriptor_ui | D008297 |
| mesh[43].is_major_topic | False |
| mesh[43].qualifier_name | |
| mesh[43].descriptor_name | Male |
| mesh[44].qualifier_ui | |
| mesh[44].descriptor_ui | D008875 |
| mesh[44].is_major_topic | False |
| mesh[44].qualifier_name | |
| mesh[44].descriptor_name | Middle Aged |
| mesh[45].qualifier_ui | Q000188 |
| mesh[45].descriptor_ui | D008175 |
| mesh[45].is_major_topic | True |
| mesh[45].qualifier_name | drug therapy |
| mesh[45].descriptor_name | Lung Neoplasms |
| mesh[46].qualifier_ui | Q000601 |
| mesh[46].descriptor_ui | D008175 |
| mesh[46].is_major_topic | True |
| mesh[46].qualifier_name | surgery |
| mesh[46].descriptor_name | Lung Neoplasms |
| mesh[47].qualifier_ui | Q000473 |
| mesh[47].descriptor_ui | D008175 |
| mesh[47].is_major_topic | True |
| mesh[47].qualifier_name | pathology |
| mesh[47].descriptor_name | Lung Neoplasms |
| mesh[48].qualifier_ui | |
| mesh[48].descriptor_ui | D000368 |
| mesh[48].is_major_topic | False |
| mesh[48].qualifier_name | |
| mesh[48].descriptor_name | Aged |
| mesh[49].qualifier_ui | Q000008 |
| mesh[49].descriptor_ui | D000911 |
| mesh[49].is_major_topic | True |
| mesh[49].qualifier_name | administration & dosage |
| mesh[49].descriptor_name | Antibodies, Monoclonal |
| type | article |
| title | Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial |
| biblio.issue | 8 |
| biblio.volume | 31 |
| biblio.last_page | 2796 |
| biblio.first_page | 2788 |
| grants[0].funder | https://openalex.org/F4320307770 |
| grants[0].award_id | |
| grants[0].funder_display_name | AstraZeneca |
| topics[0].id | https://openalex.org/T10202 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9995999932289124 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2740 |
| topics[0].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[0].display_name | Lung Cancer Diagnosis and Treatment |
| topics[1].id | https://openalex.org/T10417 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9995999932289124 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2740 |
| topics[1].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[1].display_name | Lung Cancer Treatments and Mutations |
| topics[2].id | https://openalex.org/T12334 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9991000294685364 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2730 |
| topics[2].subfield.display_name | Oncology |
| topics[2].display_name | Lung Cancer Research Studies |
| funders[0].id | https://openalex.org/F4320307770 |
| funders[0].ror | https://ror.org/04r9x1a08 |
| funders[0].display_name | AstraZeneca |
| is_xpac | False |
| apc_list.value | 9750 |
| apc_list.currency | EUR |
| apc_list.value_usd | 11690 |
| apc_paid.value | 9750 |
| apc_paid.currency | EUR |
| apc_paid.value_usd | 11690 |
| concepts[0].id | https://openalex.org/C2777742743 |
| concepts[0].level | 5 |
| concepts[0].score | 0.9429709911346436 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q19904005 |
| concepts[0].display_name | Durvalumab |
| concepts[1].id | https://openalex.org/C71924100 |
| concepts[1].level | 0 |
| concepts[1].score | 0.8297694325447083 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[1].display_name | Medicine |
| concepts[2].id | https://openalex.org/C31174226 |
| concepts[2].level | 2 |
| concepts[2].score | 0.5471124649047852 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q64855140 |
| concepts[2].display_name | Perioperative |
| concepts[3].id | https://openalex.org/C2776694085 |
| concepts[3].level | 2 |
| concepts[3].score | 0.5423768758773804 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q974135 |
| concepts[3].display_name | Chemotherapy |
| concepts[4].id | https://openalex.org/C2776256026 |
| concepts[4].level | 2 |
| concepts[4].score | 0.5420573353767395 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q47912 |
| concepts[4].display_name | Lung cancer |
| concepts[5].id | https://openalex.org/C2908647359 |
| concepts[5].level | 2 |
| concepts[5].score | 0.5237260460853577 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q2625603 |
| concepts[5].display_name | Population |
| concepts[6].id | https://openalex.org/C203092338 |
| concepts[6].level | 3 |
| concepts[6].score | 0.5147655010223389 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q1340863 |
| concepts[6].display_name | Clinical endpoint |
| concepts[7].id | https://openalex.org/C126322002 |
| concepts[7].level | 1 |
| concepts[7].score | 0.5138304829597473 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[7].display_name | Internal medicine |
| concepts[8].id | https://openalex.org/C197934379 |
| concepts[8].level | 2 |
| concepts[8].score | 0.4809798002243042 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[8].display_name | Adverse effect |
| concepts[9].id | https://openalex.org/C141071460 |
| concepts[9].level | 1 |
| concepts[9].score | 0.45743754506111145 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q40821 |
| concepts[9].display_name | Surgery |
| concepts[10].id | https://openalex.org/C31760486 |
| concepts[10].level | 3 |
| concepts[10].score | 0.452882319688797 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q7180990 |
| concepts[10].display_name | Phases of clinical research |
| concepts[11].id | https://openalex.org/C126894567 |
| concepts[11].level | 1 |
| concepts[11].score | 0.4045260548591614 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q105650 |
| concepts[11].display_name | Urology |
| concepts[12].id | https://openalex.org/C90924648 |
| concepts[12].level | 1 |
| concepts[12].score | 0.4010123014450073 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q120569 |
| concepts[12].display_name | Gastroenterology |
| concepts[13].id | https://openalex.org/C121608353 |
| concepts[13].level | 2 |
| concepts[13].score | 0.3902493417263031 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q12078 |
| concepts[13].display_name | Cancer |
| concepts[14].id | https://openalex.org/C535046627 |
| concepts[14].level | 2 |
| concepts[14].score | 0.2539157271385193 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[14].display_name | Clinical trial |
| concepts[15].id | https://openalex.org/C2777701055 |
| concepts[15].level | 3 |
| concepts[15].score | 0.12399032711982727 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q1427096 |
| concepts[15].display_name | Immunotherapy |
| concepts[16].id | https://openalex.org/C2780030458 |
| concepts[16].level | 4 |
| concepts[16].score | 0.07938209176063538 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q7041828 |
| concepts[16].display_name | Nivolumab |
| concepts[17].id | https://openalex.org/C99454951 |
| concepts[17].level | 1 |
| concepts[17].score | 0.0 |
| concepts[17].wikidata | https://www.wikidata.org/wiki/Q932068 |
| concepts[17].display_name | Environmental health |
| keywords[0].id | https://openalex.org/keywords/durvalumab |
| keywords[0].score | 0.9429709911346436 |
| keywords[0].display_name | Durvalumab |
| keywords[1].id | https://openalex.org/keywords/medicine |
| keywords[1].score | 0.8297694325447083 |
| keywords[1].display_name | Medicine |
| keywords[2].id | https://openalex.org/keywords/perioperative |
| keywords[2].score | 0.5471124649047852 |
| keywords[2].display_name | Perioperative |
| keywords[3].id | https://openalex.org/keywords/chemotherapy |
| keywords[3].score | 0.5423768758773804 |
| keywords[3].display_name | Chemotherapy |
| keywords[4].id | https://openalex.org/keywords/lung-cancer |
| keywords[4].score | 0.5420573353767395 |
| keywords[4].display_name | Lung cancer |
| keywords[5].id | https://openalex.org/keywords/population |
| keywords[5].score | 0.5237260460853577 |
| keywords[5].display_name | Population |
| keywords[6].id | https://openalex.org/keywords/clinical-endpoint |
| keywords[6].score | 0.5147655010223389 |
| keywords[6].display_name | Clinical endpoint |
| keywords[7].id | https://openalex.org/keywords/internal-medicine |
| keywords[7].score | 0.5138304829597473 |
| keywords[7].display_name | Internal medicine |
| keywords[8].id | https://openalex.org/keywords/adverse-effect |
| keywords[8].score | 0.4809798002243042 |
| keywords[8].display_name | Adverse effect |
| keywords[9].id | https://openalex.org/keywords/surgery |
| keywords[9].score | 0.45743754506111145 |
| keywords[9].display_name | Surgery |
| keywords[10].id | https://openalex.org/keywords/phases-of-clinical-research |
| keywords[10].score | 0.452882319688797 |
| keywords[10].display_name | Phases of clinical research |
| keywords[11].id | https://openalex.org/keywords/urology |
| keywords[11].score | 0.4045260548591614 |
| keywords[11].display_name | Urology |
| keywords[12].id | https://openalex.org/keywords/gastroenterology |
| keywords[12].score | 0.4010123014450073 |
| keywords[12].display_name | Gastroenterology |
| keywords[13].id | https://openalex.org/keywords/cancer |
| keywords[13].score | 0.3902493417263031 |
| keywords[13].display_name | Cancer |
| keywords[14].id | https://openalex.org/keywords/clinical-trial |
| keywords[14].score | 0.2539157271385193 |
| keywords[14].display_name | Clinical trial |
| keywords[15].id | https://openalex.org/keywords/immunotherapy |
| keywords[15].score | 0.12399032711982727 |
| keywords[15].display_name | Immunotherapy |
| keywords[16].id | https://openalex.org/keywords/nivolumab |
| keywords[16].score | 0.07938209176063538 |
| keywords[16].display_name | Nivolumab |
| language | en |
| locations[0].id | doi:10.1038/s41591-025-03746-z |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S203256638 |
| locations[0].source.issn | 1078-8956, 1546-170X |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1078-8956 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Nature Medicine |
| locations[0].source.host_organization | https://openalex.org/P4310319908 |
| locations[0].source.host_organization_name | Nature Portfolio |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310319908 |
| locations[0].license | cc-by |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Nature Medicine |
| locations[0].landing_page_url | https://doi.org/10.1038/s41591-025-03746-z |
| locations[1].id | pmid:40450142 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Nature medicine |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/40450142 |
| locations[2].id | pmh:oai:www.research.unipd.it:11577/3554520 |
| locations[2].is_oa | True |
| locations[2].source.id | https://openalex.org/S4306402448 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | Padua Research Archive (University of Padua) |
| locations[2].source.host_organization | https://openalex.org/I138689650 |
| locations[2].source.host_organization_name | University of Padua |
| locations[2].source.host_organization_lineage | https://openalex.org/I138689650 |
| locations[2].license | other-oa |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | info:eu-repo/semantics/article |
| locations[2].license_id | https://openalex.org/licenses/other-oa |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | |
| locations[2].landing_page_url | https://hdl.handle.net/11577/3554520 |
| locations[3].id | pmh:oai:pubmedcentral.nih.gov:12353838 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S2764455111 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | PubMed Central |
| locations[3].source.host_organization | https://openalex.org/I1299303238 |
| locations[3].source.host_organization_name | National Institutes of Health |
| locations[3].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[3].license | other-oa |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | Text |
| locations[3].license_id | https://openalex.org/licenses/other-oa |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | Nat Med |
| locations[3].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/12353838 |
| locations[4].id | pmh:oai:HAL:hal-05196202v1 |
| locations[4].is_oa | False |
| locations[4].source.id | https://openalex.org/S4306402512 |
| locations[4].source.issn | |
| locations[4].source.type | repository |
| locations[4].source.is_oa | False |
| locations[4].source.issn_l | |
| locations[4].source.is_core | False |
| locations[4].source.is_in_doaj | False |
| locations[4].source.display_name | HAL (Le Centre pour la Communication Scientifique Directe) |
| locations[4].source.host_organization | https://openalex.org/I1294671590 |
| locations[4].source.host_organization_name | Centre National de la Recherche Scientifique |
| locations[4].source.host_organization_lineage | https://openalex.org/I1294671590 |
| locations[4].license | |
| locations[4].pdf_url | |
| locations[4].version | submittedVersion |
| locations[4].raw_type | Journal articles |
| locations[4].license_id | |
| locations[4].is_accepted | False |
| locations[4].is_published | False |
| locations[4].raw_source_name | Nature Medicine, 2025, ⟨10.1038/s41591-025-03746-z⟩ |
| locations[4].landing_page_url | https://hal.science/hal-05196202 |
| indexed_in | crossref, pubmed |
| authorships[0].author.id | https://openalex.org/A5062198066 |
| authorships[0].author.orcid | https://orcid.org/0000-0003-3008-5407 |
| authorships[0].author.display_name | Tina Cascone |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I1343551460 |
| authorships[0].affiliations[0].raw_affiliation_string | Department of Thoracic/Head and Neck Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA. [email protected]. |
| authorships[0].institutions[0].id | https://openalex.org/I1343551460 |
| authorships[0].institutions[0].ror | https://ror.org/04twxam07 |
| authorships[0].institutions[0].type | healthcare |
| authorships[0].institutions[0].lineage | https://openalex.org/I1343551460 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | The University of Texas MD Anderson Cancer Center |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Tina Cascone |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Department of Thoracic/Head and Neck Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA. [email protected]. |
| authorships[1].author.id | https://openalex.org/A5064162399 |
| authorships[1].author.orcid | https://orcid.org/0000-0001-5218-4970 |
| authorships[1].author.display_name | Laura Bonanno |
| authorships[1].countries | IT |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I4210109345, https://openalex.org/I4210153126 |
| authorships[1].affiliations[0].raw_affiliation_string | Medical Oncology 2, Istituto Oncologico Veneto IRCCS, Padova, Italy. |
| authorships[1].affiliations[1].institution_ids | https://openalex.org/I138689650 |
| authorships[1].affiliations[1].raw_affiliation_string | Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy. |
| authorships[1].institutions[0].id | https://openalex.org/I4210153126 |
| authorships[1].institutions[0].ror | https://ror.org/04tfzc498 |
| authorships[1].institutions[0].type | healthcare |
| authorships[1].institutions[0].lineage | https://openalex.org/I4210153126 |
| authorships[1].institutions[0].country_code | IT |
| authorships[1].institutions[0].display_name | Istituti di Ricovero e Cura a Carattere Scientifico |
| authorships[1].institutions[1].id | https://openalex.org/I4210109345 |
| authorships[1].institutions[1].ror | https://ror.org/01xcjmy57 |
| authorships[1].institutions[1].type | healthcare |
| authorships[1].institutions[1].lineage | https://openalex.org/I4210109345, https://openalex.org/I4210153126 |
| authorships[1].institutions[1].country_code | IT |
| authorships[1].institutions[1].display_name | Istituto Oncologico Veneto |
| authorships[1].institutions[2].id | https://openalex.org/I138689650 |
| authorships[1].institutions[2].ror | https://ror.org/00240q980 |
| authorships[1].institutions[2].type | education |
| authorships[1].institutions[2].lineage | https://openalex.org/I138689650 |
| authorships[1].institutions[2].country_code | IT |
| authorships[1].institutions[2].display_name | University of Padua |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Laura Bonanno |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy., Medical Oncology 2, Istituto Oncologico Veneto IRCCS, Padova, Italy. |
| authorships[2].author.id | https://openalex.org/A5071449669 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-8166-7303 |
| authorships[2].author.display_name | Florian Guisier |
| authorships[2].countries | FR |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I154526488, https://openalex.org/I4210108118, https://openalex.org/I62396329 |
| authorships[2].affiliations[0].raw_affiliation_string | Univ Rouen Normandie, LITIS Lab QuantIF team EA4108, CHU Rouen, Department of Pneumology and Inserm CIC-CRB 1404, Rouen, France. |
| authorships[2].institutions[0].id | https://openalex.org/I154526488 |
| authorships[2].institutions[0].ror | https://ror.org/02vjkv261 |
| authorships[2].institutions[0].type | government |
| authorships[2].institutions[0].lineage | https://openalex.org/I154526488 |
| authorships[2].institutions[0].country_code | FR |
| authorships[2].institutions[0].display_name | Inserm |
| authorships[2].institutions[1].id | https://openalex.org/I4210108118 |
| authorships[2].institutions[1].ror | https://ror.org/01f1vfy95 |
| authorships[2].institutions[1].type | facility |
| authorships[2].institutions[1].lineage | https://openalex.org/I141576021, https://openalex.org/I4210105918, https://openalex.org/I4210108118, https://openalex.org/I62396329, https://openalex.org/I88814501 |
| authorships[2].institutions[1].country_code | FR |
| authorships[2].institutions[1].display_name | Laboratoire d'Informatique, du Traitement de l'Information et des Systèmes |
| authorships[2].institutions[2].id | https://openalex.org/I62396329 |
| authorships[2].institutions[2].ror | https://ror.org/03nhjew95 |
| authorships[2].institutions[2].type | education |
| authorships[2].institutions[2].lineage | https://openalex.org/I4210105918, https://openalex.org/I62396329 |
| authorships[2].institutions[2].country_code | FR |
| authorships[2].institutions[2].display_name | Université de Rouen Normandie |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Florian Guisier |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Univ Rouen Normandie, LITIS Lab QuantIF team EA4108, CHU Rouen, Department of Pneumology and Inserm CIC-CRB 1404, Rouen, France. |
| authorships[3].author.id | https://openalex.org/A5022853691 |
| authorships[3].author.orcid | https://orcid.org/0000-0002-3438-6170 |
| authorships[3].author.display_name | Amelia Insa |
| authorships[3].countries | ES |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I4210094406 |
| authorships[3].affiliations[0].raw_affiliation_string | Medical Oncology Department, Hospital Clínico Universitario de Valencia, Valencia, Spain. |
| authorships[3].institutions[0].id | https://openalex.org/I4210094406 |
| authorships[3].institutions[0].ror | https://ror.org/00hpnj894 |
| authorships[3].institutions[0].type | healthcare |
| authorships[3].institutions[0].lineage | https://openalex.org/I4210094406 |
| authorships[3].institutions[0].country_code | ES |
| authorships[3].institutions[0].display_name | Hospital Clínico Universitario de Valencia |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Amelia Insa |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Medical Oncology Department, Hospital Clínico Universitario de Valencia, Valencia, Spain. |
| authorships[4].author.id | https://openalex.org/A5016984274 |
| authorships[4].author.orcid | https://orcid.org/0000-0002-9964-1232 |
| authorships[4].author.display_name | Moïshe Liberman |
| authorships[4].countries | CA |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I70931966 |
| authorships[4].affiliations[0].raw_affiliation_string | Division of Thoracic Surgery, University of Montréal, Montréal, Quebec, Canada. |
| authorships[4].affiliations[1].institution_ids | https://openalex.org/I4210142730 |
| authorships[4].affiliations[1].raw_affiliation_string | CETOC - CHUM Endoscopic Tracheobronchial and Oesophageal Center, Centre Hospitalier de l'Université de Montréal, Montreal, Quebec, Canada. |
| authorships[4].institutions[0].id | https://openalex.org/I4210142730 |
| authorships[4].institutions[0].ror | https://ror.org/0410a8y51 |
| authorships[4].institutions[0].type | healthcare |
| authorships[4].institutions[0].lineage | https://openalex.org/I4210142730 |
| authorships[4].institutions[0].country_code | CA |
| authorships[4].institutions[0].display_name | Centre Hospitalier de l’Université de Montréal |
| authorships[4].institutions[1].id | https://openalex.org/I70931966 |
| authorships[4].institutions[1].ror | https://ror.org/0161xgx34 |
| authorships[4].institutions[1].type | education |
| authorships[4].institutions[1].lineage | https://openalex.org/I70931966 |
| authorships[4].institutions[1].country_code | CA |
| authorships[4].institutions[1].display_name | Université de Montréal |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Moishe Liberman |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | CETOC - CHUM Endoscopic Tracheobronchial and Oesophageal Center, Centre Hospitalier de l'Université de Montréal, Montreal, Quebec, Canada., Division of Thoracic Surgery, University of Montréal, Montréal, Quebec, Canada. |
| authorships[5].author.id | https://openalex.org/A5044886899 |
| authorships[5].author.orcid | https://orcid.org/0000-0001-8683-0969 |
| authorships[5].author.display_name | Olivier Bylicki |
| authorships[5].countries | FR |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I4210141064 |
| authorships[5].affiliations[0].raw_affiliation_string | Pneumology Department, Hôpital d'Instruction des Armées Sainte-Anne, Toulon, France. |
| authorships[5].institutions[0].id | https://openalex.org/I4210141064 |
| authorships[5].institutions[0].ror | https://ror.org/04wpkfc35 |
| authorships[5].institutions[0].type | healthcare |
| authorships[5].institutions[0].lineage | https://openalex.org/I4210141064 |
| authorships[5].institutions[0].country_code | FR |
| authorships[5].institutions[0].display_name | Hôpital d'Instruction des Armées Sainte-Anne |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Olivier Bylicki |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Pneumology Department, Hôpital d'Instruction des Armées Sainte-Anne, Toulon, France. |
| authorships[6].author.id | https://openalex.org/A5046176016 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-7755-9422 |
| authorships[6].author.display_name | Lorenzo Livi |
| authorships[6].countries | IT |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I45084792 |
| authorships[6].affiliations[0].raw_affiliation_string | Department of Radiation Oncology, University of Florence, Florence, Italy. |
| authorships[6].institutions[0].id | https://openalex.org/I45084792 |
| authorships[6].institutions[0].ror | https://ror.org/04jr1s763 |
| authorships[6].institutions[0].type | education |
| authorships[6].institutions[0].lineage | https://openalex.org/I45084792 |
| authorships[6].institutions[0].country_code | IT |
| authorships[6].institutions[0].display_name | University of Florence |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Lorenzo Livi |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Department of Radiation Oncology, University of Florence, Florence, Italy. |
| authorships[7].author.id | https://openalex.org/A5073425557 |
| authorships[7].author.orcid | https://orcid.org/0000-0002-2358-6951 |
| authorships[7].author.display_name | Thomas Egenod |
| authorships[7].countries | FR |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I4210159361 |
| authorships[7].affiliations[0].raw_affiliation_string | Department of Thoracic Oncology, Dupuytren University Hospital, Limoges, France. |
| authorships[7].institutions[0].id | https://openalex.org/I4210159361 |
| authorships[7].institutions[0].ror | https://ror.org/053385a79 |
| authorships[7].institutions[0].type | healthcare |
| authorships[7].institutions[0].lineage | https://openalex.org/I4210097159, https://openalex.org/I4210130325, https://openalex.org/I4210159361 |
| authorships[7].institutions[0].country_code | FR |
| authorships[7].institutions[0].display_name | Hôpital Dupuytren |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | Thomas Egenod |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Department of Thoracic Oncology, Dupuytren University Hospital, Limoges, France. |
| authorships[8].author.id | https://openalex.org/A5052628105 |
| authorships[8].author.orcid | https://orcid.org/0000-0002-1245-411X |
| authorships[8].author.display_name | R. Corre |
| authorships[8].countries | FR |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I4210116491 |
| authorships[8].affiliations[0].raw_affiliation_string | Department of Medical Oncology, CH de Cornouaille, Quimper, France. |
| authorships[8].institutions[0].id | https://openalex.org/I4210116491 |
| authorships[8].institutions[0].ror | https://ror.org/02pcvrc50 |
| authorships[8].institutions[0].type | healthcare |
| authorships[8].institutions[0].lineage | https://openalex.org/I4210116491 |
| authorships[8].institutions[0].country_code | FR |
| authorships[8].institutions[0].display_name | Centre Hospitalier de Cornouaille |
| authorships[8].author_position | middle |
| authorships[8].raw_author_name | Romain Corre |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Department of Medical Oncology, CH de Cornouaille, Quimper, France. |
| authorships[9].author.id | https://openalex.org/A5100638261 |
| authorships[9].author.orcid | https://orcid.org/0000-0001-5124-7132 |
| authorships[9].author.display_name | Dong‐Wan Kim |
| authorships[9].countries | KR |
| authorships[9].affiliations[0].institution_ids | https://openalex.org/I2802835388 |
| authorships[9].affiliations[0].raw_affiliation_string | Department of Internal Medicine, Seoul National University College of Medicine and Seoul National Hospital, Seoul, South Korea. |
| authorships[9].institutions[0].id | https://openalex.org/I2802835388 |
| authorships[9].institutions[0].ror | https://ror.org/01z4nnt86 |
| authorships[9].institutions[0].type | healthcare |
| authorships[9].institutions[0].lineage | https://openalex.org/I2802835388 |
| authorships[9].institutions[0].country_code | KR |
| authorships[9].institutions[0].display_name | Seoul National University Hospital |
| authorships[9].author_position | middle |
| authorships[9].raw_author_name | Dong-Wan Kim |
| authorships[9].is_corresponding | False |
| authorships[9].raw_affiliation_strings | Department of Internal Medicine, Seoul National University College of Medicine and Seoul National Hospital, Seoul, South Korea. |
| authorships[10].author.id | https://openalex.org/A5055885018 |
| authorships[10].author.orcid | https://orcid.org/0000-0003-2113-1504 |
| authorships[10].author.display_name | Rosario García-Campelo |
| authorships[10].affiliations[0].raw_affiliation_string | Hospital de A Coruña, Sergas, A Coruña, Spain. |
| authorships[10].author_position | middle |
| authorships[10].raw_author_name | Maria Rosario Garcia Campelo |
| authorships[10].is_corresponding | False |
| authorships[10].raw_affiliation_strings | Hospital de A Coruña, Sergas, A Coruña, Spain. |
| authorships[11].author.id | https://openalex.org/A5085499011 |
| authorships[11].author.orcid | https://orcid.org/0000-0001-6315-7919 |
| authorships[11].author.display_name | Mariano Provencio |
| authorships[11].countries | ES |
| authorships[11].affiliations[0].institution_ids | https://openalex.org/I4210105637 |
| authorships[11].affiliations[0].raw_affiliation_string | Puerta de Hierro University Hospital, Majadahonda, Spain. |
| authorships[11].institutions[0].id | https://openalex.org/I4210105637 |
| authorships[11].institutions[0].ror | https://ror.org/01e57nb43 |
| authorships[11].institutions[0].type | healthcare |
| authorships[11].institutions[0].lineage | https://openalex.org/I4210105637, https://openalex.org/I4210139293 |
| authorships[11].institutions[0].country_code | ES |
| authorships[11].institutions[0].display_name | Hospital Universitario Puerta de Hierro Majadahonda |
| authorships[11].author_position | middle |
| authorships[11].raw_author_name | Mariano Provencio Pulla |
| authorships[11].is_corresponding | False |
| authorships[11].raw_affiliation_strings | Puerta de Hierro University Hospital, Majadahonda, Spain. |
| authorships[12].author.id | https://openalex.org/A5011357776 |
| authorships[12].author.orcid | https://orcid.org/0000-0001-6473-786X |
| authorships[12].author.display_name | Byoung Yong Shim |
| authorships[12].countries | KR |
| authorships[12].affiliations[0].institution_ids | https://openalex.org/I4210092045 |
| authorships[12].affiliations[0].raw_affiliation_string | Department of Medical Oncology, The Catholic University of Korea, St. Vincent's Hospital, Seoul, South Korea. |
| authorships[12].institutions[0].id | https://openalex.org/I4210092045 |
| authorships[12].institutions[0].ror | https://ror.org/00msb1w96 |
| authorships[12].institutions[0].type | healthcare |
| authorships[12].institutions[0].lineage | https://openalex.org/I4210092045, https://openalex.org/I4412460286, https://openalex.org/I87111246 |
| authorships[12].institutions[0].country_code | KR |
| authorships[12].institutions[0].display_name | St. Vincent's Hospital |
| authorships[12].author_position | middle |
| authorships[12].raw_author_name | Byoung Yong Shim |
| authorships[12].is_corresponding | False |
| authorships[12].raw_affiliation_strings | Department of Medical Oncology, The Catholic University of Korea, St. Vincent's Hospital, Seoul, South Korea. |
| authorships[13].author.id | https://openalex.org/A5034761732 |
| authorships[13].author.orcid | https://orcid.org/0000-0002-4978-9212 |
| authorships[13].author.display_name | Giulio Metro |
| authorships[13].countries | IT |
| authorships[13].affiliations[0].institution_ids | https://openalex.org/I27483092 |
| authorships[13].affiliations[0].raw_affiliation_string | Santa Maria della Misericordia Hospital, University of Perugia, Perugia, Italy. |
| authorships[13].institutions[0].id | https://openalex.org/I27483092 |
| authorships[13].institutions[0].ror | https://ror.org/00x27da85 |
| authorships[13].institutions[0].type | education |
| authorships[13].institutions[0].lineage | https://openalex.org/I27483092 |
| authorships[13].institutions[0].country_code | IT |
| authorships[13].institutions[0].display_name | University of Perugia |
| authorships[13].author_position | middle |
| authorships[13].raw_author_name | Giulio Metro |
| authorships[13].is_corresponding | False |
| authorships[13].raw_affiliation_strings | Santa Maria della Misericordia Hospital, University of Perugia, Perugia, Italy. |
| authorships[14].author.id | https://openalex.org/A5030078141 |
| authorships[14].author.orcid | https://orcid.org/0000-0002-5034-1108 |
| authorships[14].author.display_name | Jaafar Bennouna |
| authorships[14].countries | FR |
| authorships[14].affiliations[0].institution_ids | https://openalex.org/I4210156825 |
| authorships[14].affiliations[0].raw_affiliation_string | Department of Medical Oncology, Hôpital Foch, Suresnes, France. |
| authorships[14].institutions[0].id | https://openalex.org/I4210156825 |
| authorships[14].institutions[0].ror | https://ror.org/058td2q88 |
| authorships[14].institutions[0].type | healthcare |
| authorships[14].institutions[0].lineage | https://openalex.org/I4210156825 |
| authorships[14].institutions[0].country_code | FR |
| authorships[14].institutions[0].display_name | Hôpital Foch |
| authorships[14].author_position | middle |
| authorships[14].raw_author_name | Jaafar Bennouna |
| authorships[14].is_corresponding | False |
| authorships[14].raw_affiliation_strings | Department of Medical Oncology, Hôpital Foch, Suresnes, France. |
| authorships[15].author.id | https://openalex.org/A5072997251 |
| authorships[15].author.orcid | https://orcid.org/0000-0002-6502-0972 |
| authorships[15].author.display_name | Agata A. Bielska |
| authorships[15].countries | US |
| authorships[15].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[15].affiliations[0].raw_affiliation_string | AstraZeneca, Waltham, MA, USA. |
| authorships[15].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[15].institutions[0].ror | https://ror.org/043cec594 |
| authorships[15].institutions[0].type | company |
| authorships[15].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[15].institutions[0].country_code | US |
| authorships[15].institutions[0].display_name | AstraZeneca (United States) |
| authorships[15].author_position | middle |
| authorships[15].raw_author_name | Agata A Bielska |
| authorships[15].is_corresponding | False |
| authorships[15].raw_affiliation_strings | AstraZeneca, Waltham, MA, USA. |
| authorships[16].author.id | https://openalex.org/A5008732453 |
| authorships[16].author.orcid | https://orcid.org/0000-0002-8479-7891 |
| authorships[16].author.display_name | Alula R. Yohannes |
| authorships[16].countries | US |
| authorships[16].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[16].affiliations[0].raw_affiliation_string | AstraZeneca, Gaithersburg, MD, USA. |
| authorships[16].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[16].institutions[0].ror | https://ror.org/043cec594 |
| authorships[16].institutions[0].type | company |
| authorships[16].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[16].institutions[0].country_code | US |
| authorships[16].institutions[0].display_name | AstraZeneca (United States) |
| authorships[16].author_position | middle |
| authorships[16].raw_author_name | Alula R Yohannes |
| authorships[16].is_corresponding | False |
| authorships[16].raw_affiliation_strings | AstraZeneca, Gaithersburg, MD, USA. |
| authorships[17].author.id | https://openalex.org/A5051718851 |
| authorships[17].author.orcid | https://orcid.org/0000-0002-0077-3113 |
| authorships[17].author.display_name | Yun He |
| authorships[17].countries | US |
| authorships[17].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[17].affiliations[0].raw_affiliation_string | AstraZeneca, Waltham, MA, USA. |
| authorships[17].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[17].institutions[0].ror | https://ror.org/043cec594 |
| authorships[17].institutions[0].type | company |
| authorships[17].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[17].institutions[0].country_code | US |
| authorships[17].institutions[0].display_name | AstraZeneca (United States) |
| authorships[17].author_position | middle |
| authorships[17].raw_author_name | Yun He |
| authorships[17].is_corresponding | False |
| authorships[17].raw_affiliation_strings | AstraZeneca, Waltham, MA, USA. |
| authorships[18].author.id | https://openalex.org/A5059378692 |
| authorships[18].author.orcid | |
| authorships[18].author.display_name | Adam Dowson |
| authorships[18].countries | GB |
| authorships[18].affiliations[0].institution_ids | https://openalex.org/I105036370 |
| authorships[18].affiliations[0].raw_affiliation_string | AstraZeneca, Cambridge, UK. |
| authorships[18].institutions[0].id | https://openalex.org/I105036370 |
| authorships[18].institutions[0].ror | https://ror.org/04r9x1a08 |
| authorships[18].institutions[0].type | company |
| authorships[18].institutions[0].lineage | https://openalex.org/I105036370 |
| authorships[18].institutions[0].country_code | GB |
| authorships[18].institutions[0].display_name | AstraZeneca (United Kingdom) |
| authorships[18].author_position | middle |
| authorships[18].raw_author_name | Adam Dowson |
| authorships[18].is_corresponding | False |
| authorships[18].raw_affiliation_strings | AstraZeneca, Cambridge, UK. |
| authorships[19].author.id | https://openalex.org/A5035685178 |
| authorships[19].author.orcid | https://orcid.org/0009-0002-7473-2725 |
| authorships[19].author.display_name | Gozde Kar |
| authorships[19].countries | GB |
| authorships[19].affiliations[0].institution_ids | https://openalex.org/I105036370 |
| authorships[19].affiliations[0].raw_affiliation_string | AstraZeneca, Cambridge, UK. |
| authorships[19].institutions[0].id | https://openalex.org/I105036370 |
| authorships[19].institutions[0].ror | https://ror.org/04r9x1a08 |
| authorships[19].institutions[0].type | company |
| authorships[19].institutions[0].lineage | https://openalex.org/I105036370 |
| authorships[19].institutions[0].country_code | GB |
| authorships[19].institutions[0].display_name | AstraZeneca (United Kingdom) |
| authorships[19].author_position | middle |
| authorships[19].raw_author_name | Gozde Kar |
| authorships[19].is_corresponding | False |
| authorships[19].raw_affiliation_strings | AstraZeneca, Cambridge, UK. |
| authorships[20].author.id | https://openalex.org/A5041696291 |
| authorships[20].author.orcid | https://orcid.org/0000-0001-6190-4802 |
| authorships[20].author.display_name | Lara McGrath |
| authorships[20].countries | US |
| authorships[20].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[20].affiliations[0].raw_affiliation_string | AstraZeneca, Waltham, MA, USA. |
| authorships[20].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[20].institutions[0].ror | https://ror.org/043cec594 |
| authorships[20].institutions[0].type | company |
| authorships[20].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[20].institutions[0].country_code | US |
| authorships[20].institutions[0].display_name | AstraZeneca (United States) |
| authorships[20].author_position | middle |
| authorships[20].raw_author_name | Lara McGrath |
| authorships[20].is_corresponding | False |
| authorships[20].raw_affiliation_strings | AstraZeneca, Waltham, MA, USA. |
| authorships[21].author.id | https://openalex.org/A5088647694 |
| authorships[21].author.orcid | https://orcid.org/0000-0002-4627-3667 |
| authorships[21].author.display_name | Rakesh Kumar |
| authorships[21].countries | US |
| authorships[21].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[21].affiliations[0].raw_affiliation_string | AstraZeneca, Gaithersburg, MD, USA. |
| authorships[21].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[21].institutions[0].ror | https://ror.org/043cec594 |
| authorships[21].institutions[0].type | company |
| authorships[21].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[21].institutions[0].country_code | US |
| authorships[21].institutions[0].display_name | AstraZeneca (United States) |
| authorships[21].author_position | middle |
| authorships[21].raw_author_name | Rakesh Kumar |
| authorships[21].is_corresponding | False |
| authorships[21].raw_affiliation_strings | AstraZeneca, Gaithersburg, MD, USA. |
| authorships[22].author.id | https://openalex.org/A5082321885 |
| authorships[22].author.orcid | https://orcid.org/0009-0006-0585-0532 |
| authorships[22].author.display_name | Italia Grenga |
| authorships[22].countries | US |
| authorships[22].affiliations[0].institution_ids | https://openalex.org/I4210150756 |
| authorships[22].affiliations[0].raw_affiliation_string | AstraZeneca, Waltham, MA, USA. |
| authorships[22].institutions[0].id | https://openalex.org/I4210150756 |
| authorships[22].institutions[0].ror | https://ror.org/043cec594 |
| authorships[22].institutions[0].type | company |
| authorships[22].institutions[0].lineage | https://openalex.org/I105036370, https://openalex.org/I4210150756 |
| authorships[22].institutions[0].country_code | US |
| authorships[22].institutions[0].display_name | AstraZeneca (United States) |
| authorships[22].author_position | middle |
| authorships[22].raw_author_name | Italia Grenga |
| authorships[22].is_corresponding | False |
| authorships[22].raw_affiliation_strings | AstraZeneca, Waltham, MA, USA. |
| authorships[23].author.id | https://openalex.org/A5049486044 |
| authorships[23].author.orcid | https://orcid.org/0000-0003-2708-1309 |
| authorships[23].author.display_name | Jonathan Spicer |
| authorships[23].countries | CA |
| authorships[23].affiliations[0].institution_ids | https://openalex.org/I5023651 |
| authorships[23].affiliations[0].raw_affiliation_string | Department of Thoracic Surgery, McGill University, Montreal, Quebec, Canada. |
| authorships[23].institutions[0].id | https://openalex.org/I5023651 |
| authorships[23].institutions[0].ror | https://ror.org/01pxwe438 |
| authorships[23].institutions[0].type | education |
| authorships[23].institutions[0].lineage | https://openalex.org/I5023651 |
| authorships[23].institutions[0].country_code | CA |
| authorships[23].institutions[0].display_name | McGill University |
| authorships[23].author_position | middle |
| authorships[23].raw_author_name | Jonathan Spicer |
| authorships[23].is_corresponding | False |
| authorships[23].raw_affiliation_strings | Department of Thoracic Surgery, McGill University, Montreal, Quebec, Canada. |
| authorships[24].author.id | https://openalex.org/A5059543131 |
| authorships[24].author.orcid | https://orcid.org/0000-0001-6925-6344 |
| authorships[24].author.display_name | Patrick M. Forde |
| authorships[24].countries | IE |
| authorships[24].affiliations[0].institution_ids | https://openalex.org/I205274468 |
| authorships[24].affiliations[0].raw_affiliation_string | Trinity St. James's Cancer Institute, Trinity College Dublin, Dublin, Ireland. |
| authorships[24].institutions[0].id | https://openalex.org/I205274468 |
| authorships[24].institutions[0].ror | https://ror.org/02tyrky19 |
| authorships[24].institutions[0].type | education |
| authorships[24].institutions[0].lineage | https://openalex.org/I205274468 |
| authorships[24].institutions[0].country_code | IE |
| authorships[24].institutions[0].display_name | Trinity College Dublin |
| authorships[24].author_position | last |
| authorships[24].raw_author_name | Patrick M Forde |
| authorships[24].is_corresponding | False |
| authorships[24].raw_affiliation_strings | Trinity St. James's Cancer Institute, Trinity College Dublin, Dublin, Ireland. |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.1038/s41591-025-03746-z |
| open_access.oa_status | hybrid |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-06-01T00:00:00 |
| display_name | Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10202 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9995999932289124 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2740 |
| primary_topic.subfield.display_name | Pulmonary and Respiratory Medicine |
| primary_topic.display_name | Lung Cancer Diagnosis and Treatment |
| related_works | https://openalex.org/W2921840556, https://openalex.org/W4389728640, https://openalex.org/W2009096079, https://openalex.org/W4241786952, https://openalex.org/W3125059937, https://openalex.org/W2995532498, https://openalex.org/W4284988128, https://openalex.org/W4312139687, https://openalex.org/W3201319284, https://openalex.org/W2145527406 |
| cited_by_count | 8 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 8 |
| locations_count | 5 |
| best_oa_location.id | doi:10.1038/s41591-025-03746-z |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S203256638 |
| best_oa_location.source.issn | 1078-8956, 1546-170X |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | 1078-8956 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | Nature Medicine |
| best_oa_location.source.host_organization | https://openalex.org/P4310319908 |
| best_oa_location.source.host_organization_name | Nature Portfolio |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310319908 |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Nature Medicine |
| best_oa_location.landing_page_url | https://doi.org/10.1038/s41591-025-03746-z |
| primary_location.id | doi:10.1038/s41591-025-03746-z |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S203256638 |
| primary_location.source.issn | 1078-8956, 1546-170X |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1078-8956 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Nature Medicine |
| primary_location.source.host_organization | https://openalex.org/P4310319908 |
| primary_location.source.host_organization_name | Nature Portfolio |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310319908 |
| primary_location.license | cc-by |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Nature Medicine |
| primary_location.landing_page_url | https://doi.org/10.1038/s41591-025-03746-z |
| publication_date | 2025-05-31 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W4400859126, https://openalex.org/W2507737385, https://openalex.org/W2228221433, https://openalex.org/W4387879971, https://openalex.org/W4379209546, https://openalex.org/W4396915845, https://openalex.org/W4223502512, https://openalex.org/W4226382697, https://openalex.org/W2123176392, https://openalex.org/W4362526763, https://openalex.org/W2902856530, https://openalex.org/W4386725951, https://openalex.org/W3196123505, https://openalex.org/W4226097966, https://openalex.org/W4402350756, https://openalex.org/W3003197494, https://openalex.org/W2776126183, https://openalex.org/W2799596353, https://openalex.org/W2982690310, https://openalex.org/W2299125010, https://openalex.org/W1975404751, https://openalex.org/W6643851160 |
| referenced_works_count | 22 |
| abstract_inverted_index.( | 107 |
| abstract_inverted_index.. | 244 |
| abstract_inverted_index.1 | 72 |
| abstract_inverted_index.= | 109, 114, 119, 124 |
| abstract_inverted_index.a | 30, 38 |
| abstract_inverted_index.n | 108, 113, 118, 123 |
| abstract_inverted_index.1, | 112, 167 |
| abstract_inverted_index.2) | 74 |
| abstract_inverted_index.2, | 117, 168 |
| abstract_inverted_index.4, | 122, 170 |
| abstract_inverted_index.II | 4 |
| abstract_inverted_index.In | 1, 102, 172, 204 |
| abstract_inverted_index.an | 211 |
| abstract_inverted_index.by | 61 |
| abstract_inverted_index.in | 165, 194, 215, 222, 229, 237 |
| abstract_inverted_index.of | 94, 232 |
| abstract_inverted_index.or | 35, 43, 69, 75 |
| abstract_inverted_index.to | 21 |
| abstract_inverted_index.1), | 34 |
| abstract_inverted_index.2), | 42 |
| abstract_inverted_index.202 | 8 |
| abstract_inverted_index.4), | 59 |
| abstract_inverted_index.4). | 79 |
| abstract_inverted_index.70; | 120 |
| abstract_inverted_index.74; | 115 |
| abstract_inverted_index.95% | 131, 136, 142, 151, 156, 162 |
| abstract_inverted_index.ADC | 212 |
| abstract_inverted_index.Arm | 111, 116, 121 |
| abstract_inverted_index.CI, | 132, 137, 143, 152, 157, 163 |
| abstract_inverted_index.and | 64, 73, 88, 96, 139, 145, 159, 169, 180, 199, 234 |
| abstract_inverted_index.mPR | 146 |
| abstract_inverted_index.pCR | 126, 218 |
| abstract_inverted_index.the | 2, 51, 103, 173, 206, 223, 238 |
| abstract_inverted_index.(Arm | 33, 41, 58, 78 |
| abstract_inverted_index.198; | 110 |
| abstract_inverted_index.54), | 125 |
| abstract_inverted_index.ADCs | 233 |
| abstract_inverted_index.CD73 | 31 |
| abstract_inverted_index.arm, | 225 |
| abstract_inverted_index.arms | 166 |
| abstract_inverted_index.lung | 16 |
| abstract_inverted_index.plus | 25, 46, 213 |
| abstract_inverted_index.rate | 87 |
| abstract_inverted_index.were | 19, 82, 128, 148, 220 |
| abstract_inverted_index.with | 10, 28, 36, 50, 67 |
| abstract_inverted_index.≥3 | 189 |
| abstract_inverted_index.(ADC) | 55 |
| abstract_inverted_index.(Arms | 71 |
| abstract_inverted_index.(mPR) | 100 |
| abstract_inverted_index.(pCR) | 86 |
| abstract_inverted_index.11/54 | 200 |
| abstract_inverted_index.20.3% | 129 |
| abstract_inverted_index.25.7% | 134 |
| abstract_inverted_index.27/74 | 195 |
| abstract_inverted_index.29/71 | 197 |
| abstract_inverted_index.35.2% | 140 |
| abstract_inverted_index.41.9% | 149 |
| abstract_inverted_index.50.0% | 154 |
| abstract_inverted_index.51/54 | 181 |
| abstract_inverted_index.63.0% | 160 |
| abstract_inverted_index.66/71 | 178 |
| abstract_inverted_index.69/74 | 176 |
| abstract_inverted_index.NKG2A | 39 |
| abstract_inverted_index.alone | 77 |
| abstract_inverted_index.first | 207 |
| abstract_inverted_index.grade | 188 |
| abstract_inverted_index.major | 97 |
| abstract_inverted_index.phase | 3 |
| abstract_inverted_index.rate. | 101 |
| abstract_inverted_index.rates | 127, 147, 219 |
| abstract_inverted_index.stage | 13 |
| abstract_inverted_index.trial | 209 |
| abstract_inverted_index.NSCLC, | 217 |
| abstract_inverted_index.TROP-2 | 52 |
| abstract_inverted_index.cancer | 17 |
| abstract_inverted_index.events | 192 |
| abstract_inverted_index.larger | 230 |
| abstract_inverted_index.safety | 174 |
| abstract_inverted_index.study, | 7 |
| abstract_inverted_index.trials | 231 |
| abstract_inverted_index.(15/74; | 130 |
| abstract_inverted_index.(18/70; | 135 |
| abstract_inverted_index.(19/54; | 141 |
| abstract_inverted_index.(20.4%) | 201 |
| abstract_inverted_index.(31/74; | 150 |
| abstract_inverted_index.(34/54; | 161 |
| abstract_inverted_index.(35/70; | 155 |
| abstract_inverted_index.(40.8%) | 198 |
| abstract_inverted_index.(94.4%) | 182 |
| abstract_inverted_index.(NSCLC) | 18 |
| abstract_inverted_index.Primary | 80 |
| abstract_inverted_index.adverse | 191 |
| abstract_inverted_index.further | 227 |
| abstract_inverted_index.highest | 221 |
| abstract_inverted_index.receive | 22 |
| abstract_inverted_index.safety; | 89 |
| abstract_inverted_index.surgery | 95 |
| abstract_inverted_index.(36.5%), | 196 |
| abstract_inverted_index.(93.0%), | 179 |
| abstract_inverted_index.(93.2%), | 177 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.Overall, | 187 |
| abstract_inverted_index.adjuvant | 65 |
| abstract_inverted_index.complete | 84 |
| abstract_inverted_index.followed | 60 |
| abstract_inverted_index.included | 92 |
| abstract_inverted_index.modified | 104 |
| abstract_inverted_index.occurred | 193 |
| abstract_inverted_index.patients | 9, 183 |
| abstract_inverted_index.platform | 6 |
| abstract_inverted_index.platinum | 48 |
| abstract_inverted_index.response | 85, 99 |
| abstract_inverted_index.setting. | 240 |
| abstract_inverted_index.surgery, | 185 |
| abstract_inverted_index.surgical | 62 |
| abstract_inverted_index.conjugate | 54 |
| abstract_inverted_index.endpoints | 81, 91 |
| abstract_inverted_index.examining | 210 |
| abstract_inverted_index.inhibitor | 32, 40 |
| abstract_inverted_index.oleclumab | 68 |
| abstract_inverted_index.patients, | 202 |
| abstract_inverted_index.resection | 63 |
| abstract_inverted_index.secondary | 90 |
| abstract_inverted_index.underwent | 184 |
| abstract_inverted_index.IIA–IIIB | 14 |
| abstract_inverted_index.NeoCOAST-2 | 5 |
| abstract_inverted_index.checkpoint | 235 |
| abstract_inverted_index.deruxtecan | 57 |
| abstract_inverted_index.durvalumab | 24, 45, 66, 76 |
| abstract_inverted_index.inhibition | 236 |
| abstract_inverted_index.oleclumab, | 29 |
| abstract_inverted_index.population | 106 |
| abstract_inverted_index.randomized | 20 |
| abstract_inverted_index.resectable | 12, 216 |
| abstract_inverted_index.untreated, | 11 |
| abstract_inverted_index.warranting | 226 |
| abstract_inverted_index.NCT05061550 | 243 |
| abstract_inverted_index.NeoCOAST-2, | 205 |
| abstract_inverted_index.datopotamab | 56 |
| abstract_inverted_index.feasibility | 93 |
| abstract_inverted_index.identifier: | 242 |
| abstract_inverted_index.monalizumab | 70 |
| abstract_inverted_index.neoadjuvant | 23, 44, 208, 239 |
| abstract_inverted_index.population, | 175 |
| abstract_inverted_index.16.0–37.6) | 138 |
| abstract_inverted_index.37.8–62.2) | 158 |
| abstract_inverted_index.48.7–75.7) | 164 |
| abstract_inverted_index.chemotherapy | 27, 49 |
| abstract_inverted_index.monalizumab, | 37 |
| abstract_inverted_index.pathological | 83, 98 |
| abstract_inverted_index.single-agent | 47 |
| abstract_inverted_index.11.8–31.2), | 133 |
| abstract_inverted_index.22.7–49.4), | 144 |
| abstract_inverted_index.30.5–53.9), | 153 |
| abstract_inverted_index.investigation | 228 |
| abstract_inverted_index.respectively. | 171, 186, 203 |
| abstract_inverted_index.non-small-cell | 15 |
| abstract_inverted_index.antibody–drug | 53 |
| abstract_inverted_index.platinum-doublet | 26 |
| abstract_inverted_index.treatment-related | 190 |
| abstract_inverted_index.ClinicalTrials.gov | 241 |
| abstract_inverted_index.intention-to-treat | 105 |
| abstract_inverted_index.chemo-immunotherapy | 214 |
| abstract_inverted_index.datopotamab-deruxtecan-containing | 224 |
| cited_by_percentile_year.max | 99 |
| cited_by_percentile_year.min | 98 |
| countries_distinct_count | 8 |
| institutions_distinct_count | 25 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.8500000238418579 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.99518244 |
| citation_normalized_percentile.is_in_top_1_percent | True |
| citation_normalized_percentile.is_in_top_10_percent | True |