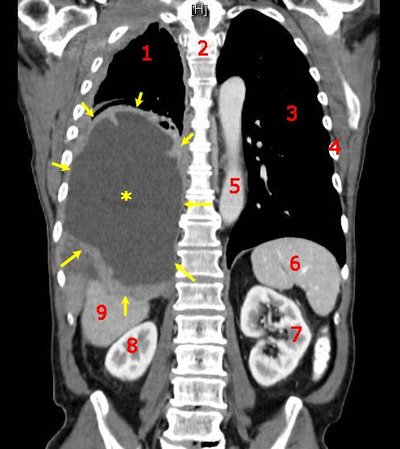
Phase II Trial of Dose-dense Pemetrexed, Gemcitabine, and Bevacizumab in Patients With Advanced, Non–Small-cell Lung Cancer Article Swipe
Bryan J. Schneider
,
Gregory P. Kalemkerian
,
Shirish M. Gadgeel
,
Manuel Valdivieso
,
Deborah Hackstock
,
Wei Chen
,
Lance K. Heilbrun
,
John C. Ruckdeschel
,
Antoinette J. Wozniak
·
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1016/j.cllc.2016.11.019
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1016/j.cllc.2016.11.019
 YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1016/j.cllc.2016.11.019
YOU?
·
· 2016
· Open Access
·
· DOI: https://doi.org/10.1016/j.cllc.2016.11.019
Related Topics
Concepts
Metadata
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1016/j.cllc.2016.11.019
- OA Status
- green
- Cited By
- 6
- References
- 16
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W2559058021
All OpenAlex metadata
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W2559058021Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1016/j.cllc.2016.11.019Digital Object Identifier
- Title
-
Phase II Trial of Dose-dense Pemetrexed, Gemcitabine, and Bevacizumab in Patients With Advanced, Non–Small-cell Lung CancerWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2016Year of publication
- Publication date
-
2016-12-02Full publication date if available
- Authors
-
Bryan J. Schneider, Gregory P. Kalemkerian, Shirish M. Gadgeel, Manuel Valdivieso, Deborah Hackstock, Wei Chen, Lance K. Heilbrun, John C. Ruckdeschel, Antoinette J. WozniakList of authors in order
- Landing page
-
https://doi.org/10.1016/j.cllc.2016.11.019Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
greenOpen access status per OpenAlex
- OA URL
-
https://www.ncbi.nlm.nih.gov/pmc/articles/6854660Direct OA link when available
- Concepts
-
Medicine, Pemetrexed, Gemcitabine, Bevacizumab, Internal medicine, Clinical endpoint, Tolerability, Lung cancer, Oncology, Neutropenia, Regimen, Phases of clinical research, Chemotherapy, Surgery, Adverse effect, Clinical trial, CisplatinTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
6Total citation count in OpenAlex
- Citations by year (recent)
-
2021: 1, 2020: 1, 2019: 2, 2017: 1Per-year citation counts (last 5 years)
- References (count)
-
16Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W2559058021 |
|---|---|
| doi | https://doi.org/10.1016/j.cllc.2016.11.019 |
| ids.doi | https://doi.org/10.1016/j.cllc.2016.11.019 |
| ids.mag | 2559058021 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/28063799 |
| ids.openalex | https://openalex.org/W2559058021 |
| fwci | 0.75554901 |
| mesh[0].qualifier_ui | |
| mesh[0].descriptor_ui | D000328 |
| mesh[0].is_major_topic | False |
| mesh[0].qualifier_name | |
| mesh[0].descriptor_name | Adult |
| mesh[1].qualifier_ui | |
| mesh[1].descriptor_ui | D000368 |
| mesh[1].is_major_topic | False |
| mesh[1].qualifier_name | |
| mesh[1].descriptor_name | Aged |
| mesh[2].qualifier_ui | |
| mesh[2].descriptor_ui | D000369 |
| mesh[2].is_major_topic | False |
| mesh[2].qualifier_name | |
| mesh[2].descriptor_name | Aged, 80 and over |
| mesh[3].qualifier_ui | Q000009 |
| mesh[3].descriptor_ui | D000971 |
| mesh[3].is_major_topic | False |
| mesh[3].qualifier_name | adverse effects |
| mesh[3].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[4].qualifier_ui | Q000627 |
| mesh[4].descriptor_ui | D000971 |
| mesh[4].is_major_topic | False |
| mesh[4].qualifier_name | therapeutic use |
| mesh[4].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[5].qualifier_ui | Q000009 |
| mesh[5].descriptor_ui | D000068258 |
| mesh[5].is_major_topic | False |
| mesh[5].qualifier_name | adverse effects |
| mesh[5].descriptor_name | Bevacizumab |
| mesh[6].qualifier_ui | Q000627 |
| mesh[6].descriptor_ui | D000068258 |
| mesh[6].is_major_topic | False |
| mesh[6].qualifier_name | therapeutic use |
| mesh[6].descriptor_name | Bevacizumab |
| mesh[7].qualifier_ui | Q000188 |
| mesh[7].descriptor_ui | D002289 |
| mesh[7].is_major_topic | False |
| mesh[7].qualifier_name | drug therapy |
| mesh[7].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[8].qualifier_ui | Q000401 |
| mesh[8].descriptor_ui | D002289 |
| mesh[8].is_major_topic | False |
| mesh[8].qualifier_name | mortality |
| mesh[8].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[9].qualifier_ui | Q000473 |
| mesh[9].descriptor_ui | D002289 |
| mesh[9].is_major_topic | False |
| mesh[9].qualifier_name | pathology |
| mesh[9].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[10].qualifier_ui | Q000009 |
| mesh[10].descriptor_ui | D003841 |
| mesh[10].is_major_topic | False |
| mesh[10].qualifier_name | adverse effects |
| mesh[10].descriptor_name | Deoxycytidine |
| mesh[11].qualifier_ui | Q000031 |
| mesh[11].descriptor_ui | D003841 |
| mesh[11].is_major_topic | False |
| mesh[11].qualifier_name | analogs & derivatives |
| mesh[11].descriptor_name | Deoxycytidine |
| mesh[12].qualifier_ui | Q000627 |
| mesh[12].descriptor_ui | D003841 |
| mesh[12].is_major_topic | False |
| mesh[12].qualifier_name | therapeutic use |
| mesh[12].descriptor_name | Deoxycytidine |
| mesh[13].qualifier_ui | |
| mesh[13].descriptor_ui | D054796 |
| mesh[13].is_major_topic | False |
| mesh[13].qualifier_name | |
| mesh[13].descriptor_name | Drug Dosage Calculations |
| mesh[14].qualifier_ui | Q000209 |
| mesh[14].descriptor_ui | D005221 |
| mesh[14].is_major_topic | False |
| mesh[14].qualifier_name | etiology |
| mesh[14].descriptor_name | Fatigue |
| mesh[15].qualifier_ui | |
| mesh[15].descriptor_ui | D005260 |
| mesh[15].is_major_topic | False |
| mesh[15].qualifier_name | |
| mesh[15].descriptor_name | Female |
| mesh[16].qualifier_ui | |
| mesh[16].descriptor_ui | D006801 |
| mesh[16].is_major_topic | False |
| mesh[16].qualifier_name | |
| mesh[16].descriptor_name | Humans |
| mesh[17].qualifier_ui | Q000178 |
| mesh[17].descriptor_ui | D008175 |
| mesh[17].is_major_topic | False |
| mesh[17].qualifier_name | diet therapy |
| mesh[17].descriptor_name | Lung Neoplasms |
| mesh[18].qualifier_ui | Q000401 |
| mesh[18].descriptor_ui | D008175 |
| mesh[18].is_major_topic | False |
| mesh[18].qualifier_name | mortality |
| mesh[18].descriptor_name | Lung Neoplasms |
| mesh[19].qualifier_ui | Q000473 |
| mesh[19].descriptor_ui | D008175 |
| mesh[19].is_major_topic | False |
| mesh[19].qualifier_name | pathology |
| mesh[19].descriptor_name | Lung Neoplasms |
| mesh[20].qualifier_ui | |
| mesh[20].descriptor_ui | D008297 |
| mesh[20].is_major_topic | False |
| mesh[20].qualifier_name | |
| mesh[20].descriptor_name | Male |
| mesh[21].qualifier_ui | |
| mesh[21].descriptor_ui | D008875 |
| mesh[21].is_major_topic | False |
| mesh[21].qualifier_name | |
| mesh[21].descriptor_name | Middle Aged |
| mesh[22].qualifier_ui | |
| mesh[22].descriptor_ui | D009367 |
| mesh[22].is_major_topic | False |
| mesh[22].qualifier_name | |
| mesh[22].descriptor_name | Neoplasm Staging |
| mesh[23].qualifier_ui | Q000209 |
| mesh[23].descriptor_ui | D009503 |
| mesh[23].is_major_topic | False |
| mesh[23].qualifier_name | etiology |
| mesh[23].descriptor_name | Neutropenia |
| mesh[24].qualifier_ui | Q000009 |
| mesh[24].descriptor_ui | D000068437 |
| mesh[24].is_major_topic | False |
| mesh[24].qualifier_name | adverse effects |
| mesh[24].descriptor_name | Pemetrexed |
| mesh[25].qualifier_ui | Q000627 |
| mesh[25].descriptor_ui | D000068437 |
| mesh[25].is_major_topic | False |
| mesh[25].qualifier_name | therapeutic use |
| mesh[25].descriptor_name | Pemetrexed |
| mesh[26].qualifier_ui | |
| mesh[26].descriptor_ui | D016019 |
| mesh[26].is_major_topic | False |
| mesh[26].qualifier_name | |
| mesh[26].descriptor_name | Survival Analysis |
| mesh[27].qualifier_ui | |
| mesh[27].descriptor_ui | D000093542 |
| mesh[27].is_major_topic | False |
| mesh[27].qualifier_name | |
| mesh[27].descriptor_name | Gemcitabine |
| mesh[28].qualifier_ui | |
| mesh[28].descriptor_ui | D000328 |
| mesh[28].is_major_topic | False |
| mesh[28].qualifier_name | |
| mesh[28].descriptor_name | Adult |
| mesh[29].qualifier_ui | |
| mesh[29].descriptor_ui | D000368 |
| mesh[29].is_major_topic | False |
| mesh[29].qualifier_name | |
| mesh[29].descriptor_name | Aged |
| mesh[30].qualifier_ui | |
| mesh[30].descriptor_ui | D000369 |
| mesh[30].is_major_topic | False |
| mesh[30].qualifier_name | |
| mesh[30].descriptor_name | Aged, 80 and over |
| mesh[31].qualifier_ui | Q000009 |
| mesh[31].descriptor_ui | D000971 |
| mesh[31].is_major_topic | False |
| mesh[31].qualifier_name | adverse effects |
| mesh[31].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[32].qualifier_ui | Q000627 |
| mesh[32].descriptor_ui | D000971 |
| mesh[32].is_major_topic | False |
| mesh[32].qualifier_name | therapeutic use |
| mesh[32].descriptor_name | Antineoplastic Combined Chemotherapy Protocols |
| mesh[33].qualifier_ui | Q000009 |
| mesh[33].descriptor_ui | D000068258 |
| mesh[33].is_major_topic | False |
| mesh[33].qualifier_name | adverse effects |
| mesh[33].descriptor_name | Bevacizumab |
| mesh[34].qualifier_ui | Q000627 |
| mesh[34].descriptor_ui | D000068258 |
| mesh[34].is_major_topic | False |
| mesh[34].qualifier_name | therapeutic use |
| mesh[34].descriptor_name | Bevacizumab |
| mesh[35].qualifier_ui | Q000188 |
| mesh[35].descriptor_ui | D002289 |
| mesh[35].is_major_topic | False |
| mesh[35].qualifier_name | drug therapy |
| mesh[35].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[36].qualifier_ui | Q000401 |
| mesh[36].descriptor_ui | D002289 |
| mesh[36].is_major_topic | False |
| mesh[36].qualifier_name | mortality |
| mesh[36].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[37].qualifier_ui | Q000473 |
| mesh[37].descriptor_ui | D002289 |
| mesh[37].is_major_topic | False |
| mesh[37].qualifier_name | pathology |
| mesh[37].descriptor_name | Carcinoma, Non-Small-Cell Lung |
| mesh[38].qualifier_ui | Q000009 |
| mesh[38].descriptor_ui | D003841 |
| mesh[38].is_major_topic | False |
| mesh[38].qualifier_name | adverse effects |
| mesh[38].descriptor_name | Deoxycytidine |
| mesh[39].qualifier_ui | Q000031 |
| mesh[39].descriptor_ui | D003841 |
| mesh[39].is_major_topic | False |
| mesh[39].qualifier_name | analogs & derivatives |
| mesh[39].descriptor_name | Deoxycytidine |
| mesh[40].qualifier_ui | Q000627 |
| mesh[40].descriptor_ui | D003841 |
| mesh[40].is_major_topic | False |
| mesh[40].qualifier_name | therapeutic use |
| mesh[40].descriptor_name | Deoxycytidine |
| mesh[41].qualifier_ui | |
| mesh[41].descriptor_ui | D054796 |
| mesh[41].is_major_topic | False |
| mesh[41].qualifier_name | |
| mesh[41].descriptor_name | Drug Dosage Calculations |
| mesh[42].qualifier_ui | Q000209 |
| mesh[42].descriptor_ui | D005221 |
| mesh[42].is_major_topic | False |
| mesh[42].qualifier_name | etiology |
| mesh[42].descriptor_name | Fatigue |
| mesh[43].qualifier_ui | |
| mesh[43].descriptor_ui | D005260 |
| mesh[43].is_major_topic | False |
| mesh[43].qualifier_name | |
| mesh[43].descriptor_name | Female |
| mesh[44].qualifier_ui | |
| mesh[44].descriptor_ui | D006801 |
| mesh[44].is_major_topic | False |
| mesh[44].qualifier_name | |
| mesh[44].descriptor_name | Humans |
| mesh[45].qualifier_ui | Q000178 |
| mesh[45].descriptor_ui | D008175 |
| mesh[45].is_major_topic | False |
| mesh[45].qualifier_name | diet therapy |
| mesh[45].descriptor_name | Lung Neoplasms |
| mesh[46].qualifier_ui | Q000401 |
| mesh[46].descriptor_ui | D008175 |
| mesh[46].is_major_topic | False |
| mesh[46].qualifier_name | mortality |
| mesh[46].descriptor_name | Lung Neoplasms |
| mesh[47].qualifier_ui | Q000473 |
| mesh[47].descriptor_ui | D008175 |
| mesh[47].is_major_topic | False |
| mesh[47].qualifier_name | pathology |
| mesh[47].descriptor_name | Lung Neoplasms |
| mesh[48].qualifier_ui | |
| mesh[48].descriptor_ui | D008297 |
| mesh[48].is_major_topic | False |
| mesh[48].qualifier_name | |
| mesh[48].descriptor_name | Male |
| mesh[49].qualifier_ui | |
| mesh[49].descriptor_ui | D008875 |
| mesh[49].is_major_topic | False |
| mesh[49].qualifier_name | |
| mesh[49].descriptor_name | Middle Aged |
| type | article |
| title | Phase II Trial of Dose-dense Pemetrexed, Gemcitabine, and Bevacizumab in Patients With Advanced, Non–Small-cell Lung Cancer |
| biblio.issue | 3 |
| biblio.volume | 18 |
| biblio.last_page | 302 |
| biblio.first_page | 299 |
| topics[0].id | https://openalex.org/T10417 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 1.0 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2740 |
| topics[0].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[0].display_name | Lung Cancer Treatments and Mutations |
| topics[1].id | https://openalex.org/T10202 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9987000226974487 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2740 |
| topics[1].subfield.display_name | Pulmonary and Respiratory Medicine |
| topics[1].display_name | Lung Cancer Diagnosis and Treatment |
| topics[2].id | https://openalex.org/T12334 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.998199999332428 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2730 |
| topics[2].subfield.display_name | Oncology |
| topics[2].display_name | Lung Cancer Research Studies |
| is_xpac | False |
| apc_list.value | 3110 |
| apc_list.currency | USD |
| apc_list.value_usd | 3110 |
| apc_paid | |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.9151824116706848 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C2777240266 |
| concepts[1].level | 4 |
| concepts[1].score | 0.8730313181877136 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q415220 |
| concepts[1].display_name | Pemetrexed |
| concepts[2].id | https://openalex.org/C2780258809 |
| concepts[2].level | 3 |
| concepts[2].score | 0.8352093696594238 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q414143 |
| concepts[2].display_name | Gemcitabine |
| concepts[3].id | https://openalex.org/C2777802072 |
| concepts[3].level | 3 |
| concepts[3].score | 0.808008074760437 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q413299 |
| concepts[3].display_name | Bevacizumab |
| concepts[4].id | https://openalex.org/C126322002 |
| concepts[4].level | 1 |
| concepts[4].score | 0.7306507229804993 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[4].display_name | Internal medicine |
| concepts[5].id | https://openalex.org/C203092338 |
| concepts[5].level | 3 |
| concepts[5].score | 0.6841628551483154 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q1340863 |
| concepts[5].display_name | Clinical endpoint |
| concepts[6].id | https://openalex.org/C2778375690 |
| concepts[6].level | 3 |
| concepts[6].score | 0.6777277588844299 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q7814214 |
| concepts[6].display_name | Tolerability |
| concepts[7].id | https://openalex.org/C2776256026 |
| concepts[7].level | 2 |
| concepts[7].score | 0.6699447631835938 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q47912 |
| concepts[7].display_name | Lung cancer |
| concepts[8].id | https://openalex.org/C143998085 |
| concepts[8].level | 1 |
| concepts[8].score | 0.6398562788963318 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q162555 |
| concepts[8].display_name | Oncology |
| concepts[9].id | https://openalex.org/C2777063308 |
| concepts[9].level | 3 |
| concepts[9].score | 0.5526488423347473 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q1435822 |
| concepts[9].display_name | Neutropenia |
| concepts[10].id | https://openalex.org/C2781413609 |
| concepts[10].level | 2 |
| concepts[10].score | 0.5490716099739075 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q7308373 |
| concepts[10].display_name | Regimen |
| concepts[11].id | https://openalex.org/C31760486 |
| concepts[11].level | 3 |
| concepts[11].score | 0.4370051622390747 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q7180990 |
| concepts[11].display_name | Phases of clinical research |
| concepts[12].id | https://openalex.org/C2776694085 |
| concepts[12].level | 2 |
| concepts[12].score | 0.36350101232528687 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q974135 |
| concepts[12].display_name | Chemotherapy |
| concepts[13].id | https://openalex.org/C141071460 |
| concepts[13].level | 1 |
| concepts[13].score | 0.3345019817352295 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q40821 |
| concepts[13].display_name | Surgery |
| concepts[14].id | https://openalex.org/C197934379 |
| concepts[14].level | 2 |
| concepts[14].score | 0.24951475858688354 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q2047938 |
| concepts[14].display_name | Adverse effect |
| concepts[15].id | https://openalex.org/C535046627 |
| concepts[15].level | 2 |
| concepts[15].score | 0.24443066120147705 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[15].display_name | Clinical trial |
| concepts[16].id | https://openalex.org/C2778239845 |
| concepts[16].level | 3 |
| concepts[16].score | 0.0891219973564148 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q412415 |
| concepts[16].display_name | Cisplatin |
| keywords[0].id | https://openalex.org/keywords/medicine |
| keywords[0].score | 0.9151824116706848 |
| keywords[0].display_name | Medicine |
| keywords[1].id | https://openalex.org/keywords/pemetrexed |
| keywords[1].score | 0.8730313181877136 |
| keywords[1].display_name | Pemetrexed |
| keywords[2].id | https://openalex.org/keywords/gemcitabine |
| keywords[2].score | 0.8352093696594238 |
| keywords[2].display_name | Gemcitabine |
| keywords[3].id | https://openalex.org/keywords/bevacizumab |
| keywords[3].score | 0.808008074760437 |
| keywords[3].display_name | Bevacizumab |
| keywords[4].id | https://openalex.org/keywords/internal-medicine |
| keywords[4].score | 0.7306507229804993 |
| keywords[4].display_name | Internal medicine |
| keywords[5].id | https://openalex.org/keywords/clinical-endpoint |
| keywords[5].score | 0.6841628551483154 |
| keywords[5].display_name | Clinical endpoint |
| keywords[6].id | https://openalex.org/keywords/tolerability |
| keywords[6].score | 0.6777277588844299 |
| keywords[6].display_name | Tolerability |
| keywords[7].id | https://openalex.org/keywords/lung-cancer |
| keywords[7].score | 0.6699447631835938 |
| keywords[7].display_name | Lung cancer |
| keywords[8].id | https://openalex.org/keywords/oncology |
| keywords[8].score | 0.6398562788963318 |
| keywords[8].display_name | Oncology |
| keywords[9].id | https://openalex.org/keywords/neutropenia |
| keywords[9].score | 0.5526488423347473 |
| keywords[9].display_name | Neutropenia |
| keywords[10].id | https://openalex.org/keywords/regimen |
| keywords[10].score | 0.5490716099739075 |
| keywords[10].display_name | Regimen |
| keywords[11].id | https://openalex.org/keywords/phases-of-clinical-research |
| keywords[11].score | 0.4370051622390747 |
| keywords[11].display_name | Phases of clinical research |
| keywords[12].id | https://openalex.org/keywords/chemotherapy |
| keywords[12].score | 0.36350101232528687 |
| keywords[12].display_name | Chemotherapy |
| keywords[13].id | https://openalex.org/keywords/surgery |
| keywords[13].score | 0.3345019817352295 |
| keywords[13].display_name | Surgery |
| keywords[14].id | https://openalex.org/keywords/adverse-effect |
| keywords[14].score | 0.24951475858688354 |
| keywords[14].display_name | Adverse effect |
| keywords[15].id | https://openalex.org/keywords/clinical-trial |
| keywords[15].score | 0.24443066120147705 |
| keywords[15].display_name | Clinical trial |
| keywords[16].id | https://openalex.org/keywords/cisplatin |
| keywords[16].score | 0.0891219973564148 |
| keywords[16].display_name | Cisplatin |
| language | en |
| locations[0].id | doi:10.1016/j.cllc.2016.11.019 |
| locations[0].is_oa | False |
| locations[0].source.id | https://openalex.org/S32518486 |
| locations[0].source.issn | 1525-7304, 1938-0690 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1525-7304 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | Clinical Lung Cancer |
| locations[0].source.host_organization | https://openalex.org/P4310320990 |
| locations[0].source.host_organization_name | Elsevier BV |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320990 |
| locations[0].license | |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Clinical Lung Cancer |
| locations[0].landing_page_url | https://doi.org/10.1016/j.cllc.2016.11.019 |
| locations[1].id | pmid:28063799 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Clinical lung cancer |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/28063799 |
| locations[2].id | pmh:oai:pubmedcentral.nih.gov:6854660 |
| locations[2].is_oa | True |
| locations[2].source.id | https://openalex.org/S2764455111 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | PubMed Central |
| locations[2].source.host_organization | https://openalex.org/I1299303238 |
| locations[2].source.host_organization_name | National Institutes of Health |
| locations[2].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | Text |
| locations[2].license_id | |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | Clin Lung Cancer |
| locations[2].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/6854660 |
| indexed_in | crossref, pubmed |
| authorships[0].author.id | https://openalex.org/A5028299369 |
| authorships[0].author.orcid | https://orcid.org/0000-0003-4189-3805 |
| authorships[0].author.display_name | Bryan J. Schneider |
| authorships[0].countries | US |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I27837315 |
| authorships[0].affiliations[0].raw_affiliation_string | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[0].institutions[0].id | https://openalex.org/I27837315 |
| authorships[0].institutions[0].ror | https://ror.org/00jmfr291 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I27837315 |
| authorships[0].institutions[0].country_code | US |
| authorships[0].institutions[0].display_name | University of Michigan |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Bryan J. Schneider |
| authorships[0].is_corresponding | True |
| authorships[0].raw_affiliation_strings | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[1].author.id | https://openalex.org/A5066306817 |
| authorships[1].author.orcid | https://orcid.org/0000-0003-2404-7420 |
| authorships[1].author.display_name | Gregory P. Kalemkerian |
| authorships[1].countries | US |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I27837315 |
| authorships[1].affiliations[0].raw_affiliation_string | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[1].institutions[0].id | https://openalex.org/I27837315 |
| authorships[1].institutions[0].ror | https://ror.org/00jmfr291 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I27837315 |
| authorships[1].institutions[0].country_code | US |
| authorships[1].institutions[0].display_name | University of Michigan |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Gregory P. Kalemkerian |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[2].author.id | https://openalex.org/A5025836672 |
| authorships[2].author.orcid | https://orcid.org/0000-0001-5240-3811 |
| authorships[2].author.display_name | Shirish M. Gadgeel |
| authorships[2].countries | US |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I185443292, https://openalex.org/I4210090317 |
| authorships[2].affiliations[0].raw_affiliation_string | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[2].institutions[0].id | https://openalex.org/I4210090317 |
| authorships[2].institutions[0].ror | https://ror.org/00ee40h97 |
| authorships[2].institutions[0].type | facility |
| authorships[2].institutions[0].lineage | https://openalex.org/I2800590384, https://openalex.org/I4210090317 |
| authorships[2].institutions[0].country_code | US |
| authorships[2].institutions[0].display_name | The Barbara Ann Karmanos Cancer Institute |
| authorships[2].institutions[1].id | https://openalex.org/I185443292 |
| authorships[2].institutions[1].ror | https://ror.org/01070mq45 |
| authorships[2].institutions[1].type | education |
| authorships[2].institutions[1].lineage | https://openalex.org/I185443292 |
| authorships[2].institutions[1].country_code | US |
| authorships[2].institutions[1].display_name | Wayne State University |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Shirish M. Gadgeel |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[3].author.id | https://openalex.org/A5111958428 |
| authorships[3].author.orcid | |
| authorships[3].author.display_name | Manuel Valdivieso |
| authorships[3].countries | US |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I27837315 |
| authorships[3].affiliations[0].raw_affiliation_string | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[3].institutions[0].id | https://openalex.org/I27837315 |
| authorships[3].institutions[0].ror | https://ror.org/00jmfr291 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I27837315 |
| authorships[3].institutions[0].country_code | US |
| authorships[3].institutions[0].display_name | University of Michigan |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Manuel Valdivieso |
| authorships[3].is_corresponding | False |
| authorships[3].raw_affiliation_strings | Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI |
| authorships[4].author.id | https://openalex.org/A5079574935 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | Deborah Hackstock |
| authorships[4].countries | US |
| authorships[4].affiliations[0].institution_ids | https://openalex.org/I185443292, https://openalex.org/I4210090317 |
| authorships[4].affiliations[0].raw_affiliation_string | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[4].institutions[0].id | https://openalex.org/I4210090317 |
| authorships[4].institutions[0].ror | https://ror.org/00ee40h97 |
| authorships[4].institutions[0].type | facility |
| authorships[4].institutions[0].lineage | https://openalex.org/I2800590384, https://openalex.org/I4210090317 |
| authorships[4].institutions[0].country_code | US |
| authorships[4].institutions[0].display_name | The Barbara Ann Karmanos Cancer Institute |
| authorships[4].institutions[1].id | https://openalex.org/I185443292 |
| authorships[4].institutions[1].ror | https://ror.org/01070mq45 |
| authorships[4].institutions[1].type | education |
| authorships[4].institutions[1].lineage | https://openalex.org/I185443292 |
| authorships[4].institutions[1].country_code | US |
| authorships[4].institutions[1].display_name | Wayne State University |
| authorships[4].author_position | middle |
| authorships[4].raw_author_name | Deborah M. Hackstock |
| authorships[4].is_corresponding | False |
| authorships[4].raw_affiliation_strings | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[5].author.id | https://openalex.org/A5100655799 |
| authorships[5].author.orcid | https://orcid.org/0000-0001-6048-1860 |
| authorships[5].author.display_name | Wei Chen |
| authorships[5].countries | US |
| authorships[5].affiliations[0].institution_ids | https://openalex.org/I185443292, https://openalex.org/I4210090317 |
| authorships[5].affiliations[0].raw_affiliation_string | Biostatistics Core, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[5].institutions[0].id | https://openalex.org/I4210090317 |
| authorships[5].institutions[0].ror | https://ror.org/00ee40h97 |
| authorships[5].institutions[0].type | facility |
| authorships[5].institutions[0].lineage | https://openalex.org/I2800590384, https://openalex.org/I4210090317 |
| authorships[5].institutions[0].country_code | US |
| authorships[5].institutions[0].display_name | The Barbara Ann Karmanos Cancer Institute |
| authorships[5].institutions[1].id | https://openalex.org/I185443292 |
| authorships[5].institutions[1].ror | https://ror.org/01070mq45 |
| authorships[5].institutions[1].type | education |
| authorships[5].institutions[1].lineage | https://openalex.org/I185443292 |
| authorships[5].institutions[1].country_code | US |
| authorships[5].institutions[1].display_name | Wayne State University |
| authorships[5].author_position | middle |
| authorships[5].raw_author_name | Wei Chen |
| authorships[5].is_corresponding | False |
| authorships[5].raw_affiliation_strings | Biostatistics Core, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[6].author.id | https://openalex.org/A5004764119 |
| authorships[6].author.orcid | https://orcid.org/0000-0002-5169-452X |
| authorships[6].author.display_name | Lance K. Heilbrun |
| authorships[6].countries | US |
| authorships[6].affiliations[0].institution_ids | https://openalex.org/I185443292, https://openalex.org/I4210090317 |
| authorships[6].affiliations[0].raw_affiliation_string | Biostatistics Core, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[6].institutions[0].id | https://openalex.org/I4210090317 |
| authorships[6].institutions[0].ror | https://ror.org/00ee40h97 |
| authorships[6].institutions[0].type | facility |
| authorships[6].institutions[0].lineage | https://openalex.org/I2800590384, https://openalex.org/I4210090317 |
| authorships[6].institutions[0].country_code | US |
| authorships[6].institutions[0].display_name | The Barbara Ann Karmanos Cancer Institute |
| authorships[6].institutions[1].id | https://openalex.org/I185443292 |
| authorships[6].institutions[1].ror | https://ror.org/01070mq45 |
| authorships[6].institutions[1].type | education |
| authorships[6].institutions[1].lineage | https://openalex.org/I185443292 |
| authorships[6].institutions[1].country_code | US |
| authorships[6].institutions[1].display_name | Wayne State University |
| authorships[6].author_position | middle |
| authorships[6].raw_author_name | Lance K. Heilbrun |
| authorships[6].is_corresponding | False |
| authorships[6].raw_affiliation_strings | Biostatistics Core, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[7].author.id | https://openalex.org/A5057026118 |
| authorships[7].author.orcid | https://orcid.org/0000-0002-7977-4257 |
| authorships[7].author.display_name | John C. Ruckdeschel |
| authorships[7].countries | US |
| authorships[7].affiliations[0].institution_ids | https://openalex.org/I4210135860 |
| authorships[7].affiliations[0].raw_affiliation_string | Synergy Cancer Center, Las Vegas, NV |
| authorships[7].institutions[0].id | https://openalex.org/I4210135860 |
| authorships[7].institutions[0].ror | https://ror.org/03ssybn81 |
| authorships[7].institutions[0].type | facility |
| authorships[7].institutions[0].lineage | https://openalex.org/I4210135860 |
| authorships[7].institutions[0].country_code | US |
| authorships[7].institutions[0].display_name | Synergy Health |
| authorships[7].author_position | middle |
| authorships[7].raw_author_name | John C. Ruckdeschel |
| authorships[7].is_corresponding | False |
| authorships[7].raw_affiliation_strings | Synergy Cancer Center, Las Vegas, NV |
| authorships[8].author.id | https://openalex.org/A5103741547 |
| authorships[8].author.orcid | |
| authorships[8].author.display_name | Antoinette J. Wozniak |
| authorships[8].countries | US |
| authorships[8].affiliations[0].institution_ids | https://openalex.org/I185443292, https://openalex.org/I4210090317 |
| authorships[8].affiliations[0].raw_affiliation_string | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| authorships[8].institutions[0].id | https://openalex.org/I4210090317 |
| authorships[8].institutions[0].ror | https://ror.org/00ee40h97 |
| authorships[8].institutions[0].type | facility |
| authorships[8].institutions[0].lineage | https://openalex.org/I2800590384, https://openalex.org/I4210090317 |
| authorships[8].institutions[0].country_code | US |
| authorships[8].institutions[0].display_name | The Barbara Ann Karmanos Cancer Institute |
| authorships[8].institutions[1].id | https://openalex.org/I185443292 |
| authorships[8].institutions[1].ror | https://ror.org/01070mq45 |
| authorships[8].institutions[1].type | education |
| authorships[8].institutions[1].lineage | https://openalex.org/I185443292 |
| authorships[8].institutions[1].country_code | US |
| authorships[8].institutions[1].display_name | Wayne State University |
| authorships[8].author_position | last |
| authorships[8].raw_author_name | Antoinette J. Wozniak |
| authorships[8].is_corresponding | False |
| authorships[8].raw_affiliation_strings | Department of Oncology, Karmanos Cancer Institute, Wayne State University, Detroit, MI |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://www.ncbi.nlm.nih.gov/pmc/articles/6854660 |
| open_access.oa_status | green |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Phase II Trial of Dose-dense Pemetrexed, Gemcitabine, and Bevacizumab in Patients With Advanced, Non–Small-cell Lung Cancer |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10417 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 1.0 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2740 |
| primary_topic.subfield.display_name | Pulmonary and Respiratory Medicine |
| primary_topic.display_name | Lung Cancer Treatments and Mutations |
| related_works | https://openalex.org/W22868739, https://openalex.org/W2127158068, https://openalex.org/W2906748950, https://openalex.org/W1977727607, https://openalex.org/W2167571882, https://openalex.org/W4226271282, https://openalex.org/W2995532498, https://openalex.org/W2033082527, https://openalex.org/W3048556543, https://openalex.org/W2320262955 |
| cited_by_count | 6 |
| counts_by_year[0].year | 2021 |
| counts_by_year[0].cited_by_count | 1 |
| counts_by_year[1].year | 2020 |
| counts_by_year[1].cited_by_count | 1 |
| counts_by_year[2].year | 2019 |
| counts_by_year[2].cited_by_count | 2 |
| counts_by_year[3].year | 2017 |
| counts_by_year[3].cited_by_count | 1 |
| locations_count | 3 |
| best_oa_location.id | pmh:oai:pubmedcentral.nih.gov:6854660 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S2764455111 |
| best_oa_location.source.issn | |
| best_oa_location.source.type | repository |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | |
| best_oa_location.source.is_core | False |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | PubMed Central |
| best_oa_location.source.host_organization | https://openalex.org/I1299303238 |
| best_oa_location.source.host_organization_name | National Institutes of Health |
| best_oa_location.source.host_organization_lineage | https://openalex.org/I1299303238 |
| best_oa_location.license | |
| best_oa_location.pdf_url | |
| best_oa_location.version | submittedVersion |
| best_oa_location.raw_type | Text |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | False |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | Clin Lung Cancer |
| best_oa_location.landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/6854660 |
| primary_location.id | doi:10.1016/j.cllc.2016.11.019 |
| primary_location.is_oa | False |
| primary_location.source.id | https://openalex.org/S32518486 |
| primary_location.source.issn | 1525-7304, 1938-0690 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1525-7304 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | Clinical Lung Cancer |
| primary_location.source.host_organization | https://openalex.org/P4310320990 |
| primary_location.source.host_organization_name | Elsevier BV |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320990 |
| primary_location.license | |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Clinical Lung Cancer |
| primary_location.landing_page_url | https://doi.org/10.1016/j.cllc.2016.11.019 |
| publication_date | 2016-12-02 |
| publication_year | 2016 |
| referenced_works | https://openalex.org/W2101760255, https://openalex.org/W1988428753, https://openalex.org/W2144418840, https://openalex.org/W2273112624, https://openalex.org/W2144660856, https://openalex.org/W2148367564, https://openalex.org/W2134693386, https://openalex.org/W2139248078, https://openalex.org/W2417902677, https://openalex.org/W6681251169, https://openalex.org/W6717190520, https://openalex.org/W2001207261, https://openalex.org/W1983700723, https://openalex.org/W2145835533, https://openalex.org/W2796537448, https://openalex.org/W4247032511 |
| referenced_works_count | 16 |
| abstract_inverted_index | |
| cited_by_percentile_year.max | 96 |
| cited_by_percentile_year.min | 89 |
| corresponding_author_ids | https://openalex.org/A5028299369 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 9 |
| corresponding_institution_ids | https://openalex.org/I27837315 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/3 |
| sustainable_development_goals[0].score | 0.8500000238418579 |
| sustainable_development_goals[0].display_name | Good health and well-being |
| citation_normalized_percentile.value | 0.77965872 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |