Rationale use of Thalidomide in erythema nodosum leprosum - A non-systematic critical analysis of published case reports Article Swipe
 YOU?
·
· 2021
· Open Access
·
· DOI: https://doi.org/10.6084/m9.figshare.14277102
YOU?
·
· 2021
· Open Access
·
· DOI: https://doi.org/10.6084/m9.figshare.14277102
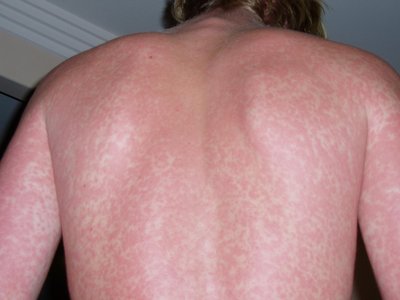
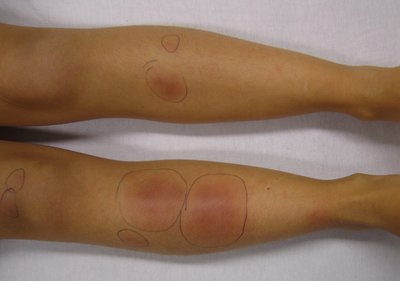
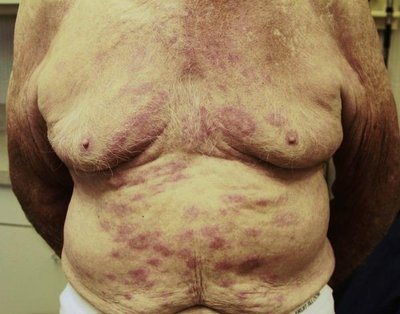
INTRODUCTION: Thalidomide is an anti- tumor necrosis factor alpha (TNF-a) drug used mainly in the management of moderate to severe form of Erythema Nodosum Leprosum (ENL). Because of its teratogenic potential it has to be used under proper supervision. Our critical analysis tries to look into the rationale with which it has been used by means of case reports on lepra reaction. METHODS: We looked for the case reports between December 2005 to June 2019 in databases like Pubmed, Embase and other relevant resources. We used search words like “erythema nodosum leprosum(ENL)”, “thalidomide”, “case report” in different combinations to get relevant reports that focus on thalidomide usage atleast once at any time point during management. The information extracted were indication of thalidomide use, dose, response, outcome, complication if any, along with all the demographic details and geographical distribution. RESULTS: We found 41 case reports eligible for analysis.The information was critically evaluated. From the analysis it was found that 7 of the case report mentioned the exact indication, 4 case report showed irrational use of thalidomide in the case of neuritis without use of steroids, 7 showed proper use of Clofazimine prior to thalidomide initiation, 26 case report showed case report of rationale dose range and in 4 case reports clofazimine was used prior to thalidomide along with the rational dose of thalidomide. CONCLUSIONS: This analysis helps to guide the rationale use of thalidomide focussing on few important points that anyone should keep in mind while managing a case of ENL.
Related Topics
- Type
- dataset
- Language
- en
- Landing Page
- https://doi.org/10.6084/m9.figshare.14277102
- OA Status
- gold
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4394224793
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4394224793Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.6084/m9.figshare.14277102Digital Object Identifier
- Title
-
Rationale use of Thalidomide in erythema nodosum leprosum - A non-systematic critical analysis of published case reportsWork title
- Type
-
datasetOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2021Year of publication
- Publication date
-
2021-01-01Full publication date if available
- Authors
-
Pugazhenthan Thangaraju, Sajıtha Venkatesan, Meenalotchini Prakash Gurunthalingam, Shoban Babu, T TamilselvanList of authors in order
- Landing page
-
https://doi.org/10.6084/m9.figshare.14277102Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.6084/m9.figshare.14277102Direct OA link when available
- Concepts
-
Thalidomide, Dermatology, Erythema nodosum, Leprosy, Medicine, Internal medicine, Multiple myeloma, DiseaseTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4394224793 |
|---|---|
| doi | https://doi.org/10.6084/m9.figshare.14277102 |
| ids.doi | https://doi.org/10.6084/m9.figshare.14277102 |
| ids.openalex | https://openalex.org/W4394224793 |
| fwci | |
| type | dataset |
| title | Rationale use of Thalidomide in erythema nodosum leprosum - A non-systematic critical analysis of published case reports |
| biblio.issue | |
| biblio.volume | |
| biblio.last_page | |
| biblio.first_page | |
| topics[0].id | https://openalex.org/T12107 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9998000264167786 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2725 |
| topics[0].subfield.display_name | Infectious Diseases |
| topics[0].display_name | Leprosy Research and Treatment |
| topics[1].id | https://openalex.org/T13374 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9797999858856201 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2711 |
| topics[1].subfield.display_name | Emergency Medicine |
| topics[1].display_name | Hematological disorders and diagnostics |
| topics[2].id | https://openalex.org/T10649 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9739000201225281 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2720 |
| topics[2].subfield.display_name | Hematology |
| topics[2].display_name | Multiple Myeloma Research and Treatments |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C2779609412 |
| concepts[0].level | 3 |
| concepts[0].score | 0.8071340322494507 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q203174 |
| concepts[0].display_name | Thalidomide |
| concepts[1].id | https://openalex.org/C16005928 |
| concepts[1].level | 1 |
| concepts[1].score | 0.6484335064888 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q171171 |
| concepts[1].display_name | Dermatology |
| concepts[2].id | https://openalex.org/C2777410594 |
| concepts[2].level | 3 |
| concepts[2].score | 0.5843886137008667 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q1363738 |
| concepts[2].display_name | Erythema nodosum |
| concepts[3].id | https://openalex.org/C2780695269 |
| concepts[3].level | 2 |
| concepts[3].score | 0.45930230617523193 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q36956 |
| concepts[3].display_name | Leprosy |
| concepts[4].id | https://openalex.org/C71924100 |
| concepts[4].level | 0 |
| concepts[4].score | 0.42565420269966125 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[4].display_name | Medicine |
| concepts[5].id | https://openalex.org/C126322002 |
| concepts[5].level | 1 |
| concepts[5].score | 0.13551971316337585 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[5].display_name | Internal medicine |
| concepts[6].id | https://openalex.org/C2776364478 |
| concepts[6].level | 2 |
| concepts[6].score | 0.0 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q467635 |
| concepts[6].display_name | Multiple myeloma |
| concepts[7].id | https://openalex.org/C2779134260 |
| concepts[7].level | 2 |
| concepts[7].score | 0.0 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q12136 |
| concepts[7].display_name | Disease |
| keywords[0].id | https://openalex.org/keywords/thalidomide |
| keywords[0].score | 0.8071340322494507 |
| keywords[0].display_name | Thalidomide |
| keywords[1].id | https://openalex.org/keywords/dermatology |
| keywords[1].score | 0.6484335064888 |
| keywords[1].display_name | Dermatology |
| keywords[2].id | https://openalex.org/keywords/erythema-nodosum |
| keywords[2].score | 0.5843886137008667 |
| keywords[2].display_name | Erythema nodosum |
| keywords[3].id | https://openalex.org/keywords/leprosy |
| keywords[3].score | 0.45930230617523193 |
| keywords[3].display_name | Leprosy |
| keywords[4].id | https://openalex.org/keywords/medicine |
| keywords[4].score | 0.42565420269966125 |
| keywords[4].display_name | Medicine |
| keywords[5].id | https://openalex.org/keywords/internal-medicine |
| keywords[5].score | 0.13551971316337585 |
| keywords[5].display_name | Internal medicine |
| language | en |
| locations[0].id | doi:10.6084/m9.figshare.14277102 |
| locations[0].is_oa | True |
| locations[0].source | |
| locations[0].license | cc-by |
| locations[0].pdf_url | |
| locations[0].version | |
| locations[0].raw_type | dataset |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | False |
| locations[0].is_published | |
| locations[0].raw_source_name | |
| locations[0].landing_page_url | https://doi.org/10.6084/m9.figshare.14277102 |
| indexed_in | datacite |
| authorships[0].author.id | https://openalex.org/A5085253964 |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | Pugazhenthan Thangaraju |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Pugazhenthan Thangaraju |
| authorships[0].is_corresponding | False |
| authorships[1].author.id | https://openalex.org/A5026841501 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | Sajıtha Venkatesan |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Sajitha Venkatesan |
| authorships[1].is_corresponding | False |
| authorships[2].author.id | https://openalex.org/A5088326769 |
| authorships[2].author.orcid | |
| authorships[2].author.display_name | Meenalotchini Prakash Gurunthalingam |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | Meenalotchini Gurunthalingam |
| authorships[2].is_corresponding | False |
| authorships[3].author.id | https://openalex.org/A5108123769 |
| authorships[3].author.orcid | |
| authorships[3].author.display_name | Shoban Babu |
| authorships[3].author_position | middle |
| authorships[3].raw_author_name | Shoban Babu |
| authorships[3].is_corresponding | False |
| authorships[4].author.id | https://openalex.org/A5002275215 |
| authorships[4].author.orcid | |
| authorships[4].author.display_name | T Tamilselvan |
| authorships[4].author_position | last |
| authorships[4].raw_author_name | Tamilselvan T |
| authorships[4].is_corresponding | False |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.6084/m9.figshare.14277102 |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Rationale use of Thalidomide in erythema nodosum leprosum - A non-systematic critical analysis of published case reports |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T06:51:31.235846 |
| primary_topic.id | https://openalex.org/T12107 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9998000264167786 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2725 |
| primary_topic.subfield.display_name | Infectious Diseases |
| primary_topic.display_name | Leprosy Research and Treatment |
| related_works | https://openalex.org/W2748952813, https://openalex.org/W3031052312, https://openalex.org/W4389568370, https://openalex.org/W3032375762, https://openalex.org/W1995515455, https://openalex.org/W2080531066, https://openalex.org/W3108674512, https://openalex.org/W1506200166, https://openalex.org/W1489783725, https://openalex.org/W2247197151 |
| cited_by_count | 0 |
| locations_count | 1 |
| best_oa_location.id | doi:10.6084/m9.figshare.14277102 |
| best_oa_location.is_oa | True |
| best_oa_location.source | |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | |
| best_oa_location.version | |
| best_oa_location.raw_type | dataset |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | False |
| best_oa_location.is_published | False |
| best_oa_location.raw_source_name | |
| best_oa_location.landing_page_url | https://doi.org/10.6084/m9.figshare.14277102 |
| primary_location.id | doi:10.6084/m9.figshare.14277102 |
| primary_location.is_oa | True |
| primary_location.source | |
| primary_location.license | cc-by |
| primary_location.pdf_url | |
| primary_location.version | |
| primary_location.raw_type | dataset |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | False |
| primary_location.is_published | False |
| primary_location.raw_source_name | |
| primary_location.landing_page_url | https://doi.org/10.6084/m9.figshare.14277102 |
| publication_date | 2021-01-01 |
| publication_year | 2021 |
| referenced_works_count | 0 |
| abstract_inverted_index.4 | 168, 207 |
| abstract_inverted_index.7 | 159, 185 |
| abstract_inverted_index.a | 247 |
| abstract_inverted_index.26 | 195 |
| abstract_inverted_index.41 | 142 |
| abstract_inverted_index.We | 64, 85, 140 |
| abstract_inverted_index.an | 4 |
| abstract_inverted_index.at | 110 |
| abstract_inverted_index.be | 35 |
| abstract_inverted_index.by | 55 |
| abstract_inverted_index.if | 128 |
| abstract_inverted_index.in | 14, 76, 96, 176, 206, 243 |
| abstract_inverted_index.is | 3 |
| abstract_inverted_index.it | 32, 51, 155 |
| abstract_inverted_index.of | 17, 22, 28, 57, 121, 160, 174, 179, 183, 189, 201, 221, 232, 249 |
| abstract_inverted_index.on | 60, 105, 235 |
| abstract_inverted_index.to | 19, 34, 44, 73, 99, 192, 214, 227 |
| abstract_inverted_index.Our | 40 |
| abstract_inverted_index.The | 116 |
| abstract_inverted_index.all | 132 |
| abstract_inverted_index.and | 81, 136, 205 |
| abstract_inverted_index.any | 111 |
| abstract_inverted_index.few | 236 |
| abstract_inverted_index.for | 66, 146 |
| abstract_inverted_index.get | 100 |
| abstract_inverted_index.has | 33, 52 |
| abstract_inverted_index.its | 29 |
| abstract_inverted_index.the | 15, 47, 67, 133, 153, 161, 165, 177, 218, 229 |
| abstract_inverted_index.use | 173, 182, 188, 231 |
| abstract_inverted_index.was | 149, 156, 211 |
| abstract_inverted_index.2005 | 72 |
| abstract_inverted_index.2019 | 75 |
| abstract_inverted_index.ENL. | 250 |
| abstract_inverted_index.From | 152 |
| abstract_inverted_index.June | 74 |
| abstract_inverted_index.This | 224 |
| abstract_inverted_index.any, | 129 |
| abstract_inverted_index.been | 53 |
| abstract_inverted_index.case | 58, 68, 143, 162, 169, 178, 196, 199, 208, 248 |
| abstract_inverted_index.dose | 203, 220 |
| abstract_inverted_index.drug | 11 |
| abstract_inverted_index.form | 21 |
| abstract_inverted_index.into | 46 |
| abstract_inverted_index.keep | 242 |
| abstract_inverted_index.like | 78, 89 |
| abstract_inverted_index.look | 45 |
| abstract_inverted_index.mind | 244 |
| abstract_inverted_index.once | 109 |
| abstract_inverted_index.that | 103, 158, 239 |
| abstract_inverted_index.time | 112 |
| abstract_inverted_index.use, | 123 |
| abstract_inverted_index.used | 12, 36, 54, 86, 212 |
| abstract_inverted_index.were | 119 |
| abstract_inverted_index.with | 49, 131, 217 |
| abstract_inverted_index.along | 130, 216 |
| abstract_inverted_index.alpha | 9 |
| abstract_inverted_index.anti- | 5 |
| abstract_inverted_index.dose, | 124 |
| abstract_inverted_index.exact | 166 |
| abstract_inverted_index.focus | 104 |
| abstract_inverted_index.found | 141, 157 |
| abstract_inverted_index.guide | 228 |
| abstract_inverted_index.helps | 226 |
| abstract_inverted_index.lepra | 61 |
| abstract_inverted_index.means | 56 |
| abstract_inverted_index.other | 82 |
| abstract_inverted_index.point | 113 |
| abstract_inverted_index.prior | 191, 213 |
| abstract_inverted_index.range | 204 |
| abstract_inverted_index.tries | 43 |
| abstract_inverted_index.tumor | 6 |
| abstract_inverted_index.under | 37 |
| abstract_inverted_index.usage | 107 |
| abstract_inverted_index.which | 50 |
| abstract_inverted_index.while | 245 |
| abstract_inverted_index.words | 88 |
| abstract_inverted_index.(ENL). | 26 |
| abstract_inverted_index.Embase | 80 |
| abstract_inverted_index.anyone | 240 |
| abstract_inverted_index.during | 114 |
| abstract_inverted_index.factor | 8 |
| abstract_inverted_index.looked | 65 |
| abstract_inverted_index.mainly | 13 |
| abstract_inverted_index.points | 238 |
| abstract_inverted_index.proper | 38, 187 |
| abstract_inverted_index.report | 163, 170, 197, 200 |
| abstract_inverted_index.search | 87 |
| abstract_inverted_index.severe | 20 |
| abstract_inverted_index.should | 241 |
| abstract_inverted_index.showed | 171, 186, 198 |
| abstract_inverted_index.(TNF-a) | 10 |
| abstract_inverted_index.Because | 27 |
| abstract_inverted_index.Nodosum | 24 |
| abstract_inverted_index.Pubmed, | 79 |
| abstract_inverted_index.atleast | 108 |
| abstract_inverted_index.between | 70 |
| abstract_inverted_index.details | 135 |
| abstract_inverted_index.nodosum | 91 |
| abstract_inverted_index.reports | 59, 69, 102, 144, 209 |
| abstract_inverted_index.without | 181 |
| abstract_inverted_index.“case | 94 |
| abstract_inverted_index.Abstract | 0 |
| abstract_inverted_index.December | 71 |
| abstract_inverted_index.Erythema | 23 |
| abstract_inverted_index.Leprosum | 25 |
| abstract_inverted_index.METHODS: | 63 |
| abstract_inverted_index.RESULTS: | 139 |
| abstract_inverted_index.analysis | 42, 154, 225 |
| abstract_inverted_index.critical | 41 |
| abstract_inverted_index.eligible | 145 |
| abstract_inverted_index.managing | 246 |
| abstract_inverted_index.moderate | 18 |
| abstract_inverted_index.necrosis | 7 |
| abstract_inverted_index.neuritis | 180 |
| abstract_inverted_index.outcome, | 126 |
| abstract_inverted_index.rational | 219 |
| abstract_inverted_index.relevant | 83, 101 |
| abstract_inverted_index.databases | 77 |
| abstract_inverted_index.different | 97 |
| abstract_inverted_index.extracted | 118 |
| abstract_inverted_index.focussing | 234 |
| abstract_inverted_index.important | 237 |
| abstract_inverted_index.mentioned | 164 |
| abstract_inverted_index.potential | 31 |
| abstract_inverted_index.rationale | 48, 202, 230 |
| abstract_inverted_index.reaction. | 62 |
| abstract_inverted_index.report” | 95 |
| abstract_inverted_index.response, | 125 |
| abstract_inverted_index.steroids, | 184 |
| abstract_inverted_index.critically | 150 |
| abstract_inverted_index.evaluated. | 151 |
| abstract_inverted_index.indication | 120 |
| abstract_inverted_index.irrational | 172 |
| abstract_inverted_index.management | 16 |
| abstract_inverted_index.resources. | 84 |
| abstract_inverted_index.Clofazimine | 190 |
| abstract_inverted_index.Thalidomide | 2 |
| abstract_inverted_index.clofazimine | 210 |
| abstract_inverted_index.demographic | 134 |
| abstract_inverted_index.indication, | 167 |
| abstract_inverted_index.information | 117, 148 |
| abstract_inverted_index.initiation, | 194 |
| abstract_inverted_index.management. | 115 |
| abstract_inverted_index.teratogenic | 30 |
| abstract_inverted_index.thalidomide | 106, 122, 175, 193, 215, 233 |
| abstract_inverted_index.“erythema | 90 |
| abstract_inverted_index.CONCLUSIONS: | 223 |
| abstract_inverted_index.analysis.The | 147 |
| abstract_inverted_index.combinations | 98 |
| abstract_inverted_index.complication | 127 |
| abstract_inverted_index.geographical | 137 |
| abstract_inverted_index.supervision. | 39 |
| abstract_inverted_index.thalidomide. | 222 |
| abstract_inverted_index.INTRODUCTION: | 1 |
| abstract_inverted_index.distribution. | 138 |
| abstract_inverted_index.leprosum(ENL)”, | 92 |
| abstract_inverted_index.“thalidomide”, | 93 |
| cited_by_percentile_year | |
| countries_distinct_count | 0 |
| institutions_distinct_count | 5 |
| citation_normalized_percentile |