Sustained-release oral dalfampridine appears to have no impact on upper extremity function in people with multiple sclerosis: a randomized controlled trial Article Swipe
 YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1177/17562864251321696
YOU?
·
· 2025
· Open Access
·
· DOI: https://doi.org/10.1177/17562864251321696
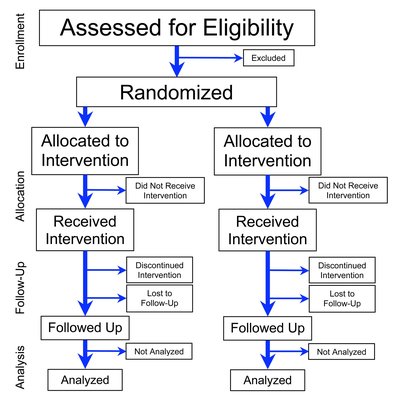
Background: Upper limb dysfunction is common in people with multiple sclerosis (pwMS), significantly affecting daily activities and quality of life. While dalfampridine has shown efficacy in improving gait in pwMS, its impact on upper extremity function remains unclear. Objectives: To evaluate the effect of sustained-release oral dalfampridine on upper extremity function in pwMS. Design: A randomized, placebo-controlled trial. Methods: In all, 30 pwMS were randomized to receive either dalfampridine (10 mg twice daily) or a placebo for 2 weeks. Upper extremity function was assessed at baseline, after 1 week, after 2 weeks of treatment, and 2 weeks post-treatment using clinical tests (9-Hole Peg Test, Box, and Block Test, peak isometric grip force, 2-point discrimination) and self-reported questionnaires (disabilities of the arm, shoulder and hand, ability measure of the hand, Manual Ability Measurement 36). Data were analyzed using repeated-measures analysis of variance to evaluate group × time interactions. Results: No significant group × time interactions were observed across clinical or self-reported outcomes. Both groups exhibited similar trends over time, with no measurable improvements in upper extremity dexterity, strength, or perceived function attributable to dalfampridine. Conclusion: Sustained-release dalfampridine does not appear to improve upper extremity function in pwMS, highlighting its limitations beyond gait-related benefits. These findings underscore the need for further research to explore alternative treatments targeting upper limb dysfunction in this population. Trial registration: ClinicalTrials.gov NCT02259361.
Related Topics
- Type
- article
- Language
- en
- Landing Page
- https://doi.org/10.1177/17562864251321696
- OA Status
- gold
- References
- 29
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W4407844088
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W4407844088Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1177/17562864251321696Digital Object Identifier
- Title
-
Sustained-release oral dalfampridine appears to have no impact on upper extremity function in people with multiple sclerosis: a randomized controlled trialWork title
- Type
-
articleOpenAlex work type
- Language
-
enPrimary language
- Publication year
-
2025Year of publication
- Publication date
-
2025-01-01Full publication date if available
- Authors
-
Shay Menascu, Lior Frid, Alon KalronList of authors in order
- Landing page
-
https://doi.org/10.1177/17562864251321696Publisher landing page
- Open access
-
YesWhether a free full text is available
- OA status
-
goldOpen access status per OpenAlex
- OA URL
-
https://doi.org/10.1177/17562864251321696Direct OA link when available
- Concepts
-
Medicine, Randomized controlled trial, Physical therapy, Placebo, Physical medicine and rehabilitation, Isometric exercise, Grip strength, Quality of life (healthcare), Upper limb, Clinical trial, Multiple sclerosis, Clinical endpoint, Population, Repeated measures design, Internal medicine, Alternative medicine, Statistics, Pathology, Environmental health, Nursing, Mathematics, PsychiatryTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
0Total citation count in OpenAlex
- References (count)
-
29Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W4407844088 |
|---|---|
| doi | https://doi.org/10.1177/17562864251321696 |
| ids.doi | https://doi.org/10.1177/17562864251321696 |
| ids.pmid | https://pubmed.ncbi.nlm.nih.gov/39990866 |
| ids.openalex | https://openalex.org/W4407844088 |
| fwci | 0.0 |
| type | article |
| title | Sustained-release oral dalfampridine appears to have no impact on upper extremity function in people with multiple sclerosis: a randomized controlled trial |
| biblio.issue | |
| biblio.volume | 18 |
| biblio.last_page | 17562864251321696 |
| biblio.first_page | 17562864251321696 |
| topics[0].id | https://openalex.org/T10137 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9995999932289124 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2734 |
| topics[0].subfield.display_name | Pathology and Forensic Medicine |
| topics[0].display_name | Multiple Sclerosis Research Studies |
| topics[1].id | https://openalex.org/T11735 |
| topics[1].field.id | https://openalex.org/fields/27 |
| topics[1].field.display_name | Medicine |
| topics[1].score | 0.9851999878883362 |
| topics[1].domain.id | https://openalex.org/domains/4 |
| topics[1].domain.display_name | Health Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/2728 |
| topics[1].subfield.display_name | Neurology |
| topics[1].display_name | Peripheral Neuropathies and Disorders |
| topics[2].id | https://openalex.org/T11616 |
| topics[2].field.id | https://openalex.org/fields/27 |
| topics[2].field.display_name | Medicine |
| topics[2].score | 0.9786999821662903 |
| topics[2].domain.id | https://openalex.org/domains/4 |
| topics[2].domain.display_name | Health Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/2720 |
| topics[2].subfield.display_name | Hematology |
| topics[2].display_name | Autoimmune and Inflammatory Disorders Research |
| is_xpac | False |
| apc_list.value | 2300 |
| apc_list.currency | USD |
| apc_list.value_usd | 2300 |
| apc_paid.value | 2300 |
| apc_paid.currency | USD |
| apc_paid.value_usd | 2300 |
| concepts[0].id | https://openalex.org/C71924100 |
| concepts[0].level | 0 |
| concepts[0].score | 0.8968625068664551 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q11190 |
| concepts[0].display_name | Medicine |
| concepts[1].id | https://openalex.org/C168563851 |
| concepts[1].level | 2 |
| concepts[1].score | 0.7001403570175171 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q1436668 |
| concepts[1].display_name | Randomized controlled trial |
| concepts[2].id | https://openalex.org/C1862650 |
| concepts[2].level | 1 |
| concepts[2].score | 0.6318199634552002 |
| concepts[2].wikidata | https://www.wikidata.org/wiki/Q186005 |
| concepts[2].display_name | Physical therapy |
| concepts[3].id | https://openalex.org/C27081682 |
| concepts[3].level | 3 |
| concepts[3].score | 0.6073768734931946 |
| concepts[3].wikidata | https://www.wikidata.org/wiki/Q269829 |
| concepts[3].display_name | Placebo |
| concepts[4].id | https://openalex.org/C99508421 |
| concepts[4].level | 1 |
| concepts[4].score | 0.6067383289337158 |
| concepts[4].wikidata | https://www.wikidata.org/wiki/Q2678675 |
| concepts[4].display_name | Physical medicine and rehabilitation |
| concepts[5].id | https://openalex.org/C103486182 |
| concepts[5].level | 2 |
| concepts[5].score | 0.5414635539054871 |
| concepts[5].wikidata | https://www.wikidata.org/wiki/Q1216236 |
| concepts[5].display_name | Isometric exercise |
| concepts[6].id | https://openalex.org/C2777433710 |
| concepts[6].level | 2 |
| concepts[6].score | 0.5288333296775818 |
| concepts[6].wikidata | https://www.wikidata.org/wiki/Q5609571 |
| concepts[6].display_name | Grip strength |
| concepts[7].id | https://openalex.org/C2779951463 |
| concepts[7].level | 2 |
| concepts[7].score | 0.5115788578987122 |
| concepts[7].wikidata | https://www.wikidata.org/wiki/Q7268788 |
| concepts[7].display_name | Quality of life (healthcare) |
| concepts[8].id | https://openalex.org/C2776660947 |
| concepts[8].level | 2 |
| concepts[8].score | 0.4880024492740631 |
| concepts[8].wikidata | https://www.wikidata.org/wiki/Q841423 |
| concepts[8].display_name | Upper limb |
| concepts[9].id | https://openalex.org/C535046627 |
| concepts[9].level | 2 |
| concepts[9].score | 0.4857619106769562 |
| concepts[9].wikidata | https://www.wikidata.org/wiki/Q30612 |
| concepts[9].display_name | Clinical trial |
| concepts[10].id | https://openalex.org/C2780640218 |
| concepts[10].level | 2 |
| concepts[10].score | 0.46972978115081787 |
| concepts[10].wikidata | https://www.wikidata.org/wiki/Q8277 |
| concepts[10].display_name | Multiple sclerosis |
| concepts[11].id | https://openalex.org/C203092338 |
| concepts[11].level | 3 |
| concepts[11].score | 0.44890493154525757 |
| concepts[11].wikidata | https://www.wikidata.org/wiki/Q1340863 |
| concepts[11].display_name | Clinical endpoint |
| concepts[12].id | https://openalex.org/C2908647359 |
| concepts[12].level | 2 |
| concepts[12].score | 0.4455142319202423 |
| concepts[12].wikidata | https://www.wikidata.org/wiki/Q2625603 |
| concepts[12].display_name | Population |
| concepts[13].id | https://openalex.org/C102959455 |
| concepts[13].level | 2 |
| concepts[13].score | 0.4114600121974945 |
| concepts[13].wikidata | https://www.wikidata.org/wiki/Q7313954 |
| concepts[13].display_name | Repeated measures design |
| concepts[14].id | https://openalex.org/C126322002 |
| concepts[14].level | 1 |
| concepts[14].score | 0.14245137572288513 |
| concepts[14].wikidata | https://www.wikidata.org/wiki/Q11180 |
| concepts[14].display_name | Internal medicine |
| concepts[15].id | https://openalex.org/C204787440 |
| concepts[15].level | 2 |
| concepts[15].score | 0.08400645852088928 |
| concepts[15].wikidata | https://www.wikidata.org/wiki/Q188504 |
| concepts[15].display_name | Alternative medicine |
| concepts[16].id | https://openalex.org/C105795698 |
| concepts[16].level | 1 |
| concepts[16].score | 0.0 |
| concepts[16].wikidata | https://www.wikidata.org/wiki/Q12483 |
| concepts[16].display_name | Statistics |
| concepts[17].id | https://openalex.org/C142724271 |
| concepts[17].level | 1 |
| concepts[17].score | 0.0 |
| concepts[17].wikidata | https://www.wikidata.org/wiki/Q7208 |
| concepts[17].display_name | Pathology |
| concepts[18].id | https://openalex.org/C99454951 |
| concepts[18].level | 1 |
| concepts[18].score | 0.0 |
| concepts[18].wikidata | https://www.wikidata.org/wiki/Q932068 |
| concepts[18].display_name | Environmental health |
| concepts[19].id | https://openalex.org/C159110408 |
| concepts[19].level | 1 |
| concepts[19].score | 0.0 |
| concepts[19].wikidata | https://www.wikidata.org/wiki/Q121176 |
| concepts[19].display_name | Nursing |
| concepts[20].id | https://openalex.org/C33923547 |
| concepts[20].level | 0 |
| concepts[20].score | 0.0 |
| concepts[20].wikidata | https://www.wikidata.org/wiki/Q395 |
| concepts[20].display_name | Mathematics |
| concepts[21].id | https://openalex.org/C118552586 |
| concepts[21].level | 1 |
| concepts[21].score | 0.0 |
| concepts[21].wikidata | https://www.wikidata.org/wiki/Q7867 |
| concepts[21].display_name | Psychiatry |
| keywords[0].id | https://openalex.org/keywords/medicine |
| keywords[0].score | 0.8968625068664551 |
| keywords[0].display_name | Medicine |
| keywords[1].id | https://openalex.org/keywords/randomized-controlled-trial |
| keywords[1].score | 0.7001403570175171 |
| keywords[1].display_name | Randomized controlled trial |
| keywords[2].id | https://openalex.org/keywords/physical-therapy |
| keywords[2].score | 0.6318199634552002 |
| keywords[2].display_name | Physical therapy |
| keywords[3].id | https://openalex.org/keywords/placebo |
| keywords[3].score | 0.6073768734931946 |
| keywords[3].display_name | Placebo |
| keywords[4].id | https://openalex.org/keywords/physical-medicine-and-rehabilitation |
| keywords[4].score | 0.6067383289337158 |
| keywords[4].display_name | Physical medicine and rehabilitation |
| keywords[5].id | https://openalex.org/keywords/isometric-exercise |
| keywords[5].score | 0.5414635539054871 |
| keywords[5].display_name | Isometric exercise |
| keywords[6].id | https://openalex.org/keywords/grip-strength |
| keywords[6].score | 0.5288333296775818 |
| keywords[6].display_name | Grip strength |
| keywords[7].id | https://openalex.org/keywords/quality-of-life |
| keywords[7].score | 0.5115788578987122 |
| keywords[7].display_name | Quality of life (healthcare) |
| keywords[8].id | https://openalex.org/keywords/upper-limb |
| keywords[8].score | 0.4880024492740631 |
| keywords[8].display_name | Upper limb |
| keywords[9].id | https://openalex.org/keywords/clinical-trial |
| keywords[9].score | 0.4857619106769562 |
| keywords[9].display_name | Clinical trial |
| keywords[10].id | https://openalex.org/keywords/multiple-sclerosis |
| keywords[10].score | 0.46972978115081787 |
| keywords[10].display_name | Multiple sclerosis |
| keywords[11].id | https://openalex.org/keywords/clinical-endpoint |
| keywords[11].score | 0.44890493154525757 |
| keywords[11].display_name | Clinical endpoint |
| keywords[12].id | https://openalex.org/keywords/population |
| keywords[12].score | 0.4455142319202423 |
| keywords[12].display_name | Population |
| keywords[13].id | https://openalex.org/keywords/repeated-measures-design |
| keywords[13].score | 0.4114600121974945 |
| keywords[13].display_name | Repeated measures design |
| keywords[14].id | https://openalex.org/keywords/internal-medicine |
| keywords[14].score | 0.14245137572288513 |
| keywords[14].display_name | Internal medicine |
| keywords[15].id | https://openalex.org/keywords/alternative-medicine |
| keywords[15].score | 0.08400645852088928 |
| keywords[15].display_name | Alternative medicine |
| language | en |
| locations[0].id | doi:10.1177/17562864251321696 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S7261370 |
| locations[0].source.issn | 1756-2856, 1756-2864 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | True |
| locations[0].source.issn_l | 1756-2856 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | True |
| locations[0].source.display_name | Therapeutic Advances in Neurological Disorders |
| locations[0].source.host_organization | https://openalex.org/P4310320017 |
| locations[0].source.host_organization_name | SAGE Publishing |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310320017 |
| locations[0].source.host_organization_lineage_names | SAGE Publishing |
| locations[0].license | |
| locations[0].pdf_url | |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | Therapeutic Advances in Neurological Disorders |
| locations[0].landing_page_url | https://doi.org/10.1177/17562864251321696 |
| locations[1].id | pmid:39990866 |
| locations[1].is_oa | False |
| locations[1].source.id | https://openalex.org/S4306525036 |
| locations[1].source.issn | |
| locations[1].source.type | repository |
| locations[1].source.is_oa | False |
| locations[1].source.issn_l | |
| locations[1].source.is_core | False |
| locations[1].source.is_in_doaj | False |
| locations[1].source.display_name | PubMed |
| locations[1].source.host_organization | https://openalex.org/I1299303238 |
| locations[1].source.host_organization_name | National Institutes of Health |
| locations[1].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[1].license | |
| locations[1].pdf_url | |
| locations[1].version | publishedVersion |
| locations[1].raw_type | |
| locations[1].license_id | |
| locations[1].is_accepted | True |
| locations[1].is_published | True |
| locations[1].raw_source_name | Therapeutic advances in neurological disorders |
| locations[1].landing_page_url | https://pubmed.ncbi.nlm.nih.gov/39990866 |
| locations[2].id | pmh:oai:doaj.org/article:9d3c053a14c94c87838c86e73a1b4a5e |
| locations[2].is_oa | False |
| locations[2].source.id | https://openalex.org/S4306401280 |
| locations[2].source.issn | |
| locations[2].source.type | repository |
| locations[2].source.is_oa | False |
| locations[2].source.issn_l | |
| locations[2].source.is_core | False |
| locations[2].source.is_in_doaj | False |
| locations[2].source.display_name | DOAJ (DOAJ: Directory of Open Access Journals) |
| locations[2].source.host_organization | |
| locations[2].source.host_organization_name | |
| locations[2].license | |
| locations[2].pdf_url | |
| locations[2].version | submittedVersion |
| locations[2].raw_type | article |
| locations[2].license_id | |
| locations[2].is_accepted | False |
| locations[2].is_published | False |
| locations[2].raw_source_name | Therapeutic Advances in Neurological Disorders, Vol 18 (2025) |
| locations[2].landing_page_url | https://doaj.org/article/9d3c053a14c94c87838c86e73a1b4a5e |
| locations[3].id | pmh:oai:pubmedcentral.nih.gov:11846113 |
| locations[3].is_oa | True |
| locations[3].source.id | https://openalex.org/S2764455111 |
| locations[3].source.issn | |
| locations[3].source.type | repository |
| locations[3].source.is_oa | False |
| locations[3].source.issn_l | |
| locations[3].source.is_core | False |
| locations[3].source.is_in_doaj | False |
| locations[3].source.display_name | PubMed Central |
| locations[3].source.host_organization | https://openalex.org/I1299303238 |
| locations[3].source.host_organization_name | National Institutes of Health |
| locations[3].source.host_organization_lineage | https://openalex.org/I1299303238 |
| locations[3].license | other-oa |
| locations[3].pdf_url | |
| locations[3].version | submittedVersion |
| locations[3].raw_type | Text |
| locations[3].license_id | https://openalex.org/licenses/other-oa |
| locations[3].is_accepted | False |
| locations[3].is_published | False |
| locations[3].raw_source_name | Ther Adv Neurol Disord |
| locations[3].landing_page_url | https://www.ncbi.nlm.nih.gov/pmc/articles/11846113 |
| indexed_in | crossref, doaj, pubmed |
| authorships[0].author.id | https://openalex.org/A5036220371 |
| authorships[0].author.orcid | https://orcid.org/0000-0001-9109-6818 |
| authorships[0].author.display_name | Shay Menascu |
| authorships[0].countries | IL |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I2799810450 |
| authorships[0].affiliations[0].raw_affiliation_string | Multiple Sclerosis Center, Sheba Medical Center, Tel-Hashomer, Israel |
| authorships[0].institutions[0].id | https://openalex.org/I2799810450 |
| authorships[0].institutions[0].ror | https://ror.org/020rzx487 |
| authorships[0].institutions[0].type | healthcare |
| authorships[0].institutions[0].lineage | https://openalex.org/I2799810450 |
| authorships[0].institutions[0].country_code | IL |
| authorships[0].institutions[0].display_name | Sheba Medical Center |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | Shay Menascu |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Multiple Sclerosis Center, Sheba Medical Center, Tel-Hashomer, Israel |
| authorships[1].author.id | https://openalex.org/A5068194593 |
| authorships[1].author.orcid | https://orcid.org/0000-0003-0289-2340 |
| authorships[1].author.display_name | Lior Frid |
| authorships[1].countries | IL |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I2799810450 |
| authorships[1].affiliations[0].raw_affiliation_string | Multiple Sclerosis Center, Sheba Medical Center, Tel-Hashomer, Israel |
| authorships[1].institutions[0].id | https://openalex.org/I2799810450 |
| authorships[1].institutions[0].ror | https://ror.org/020rzx487 |
| authorships[1].institutions[0].type | healthcare |
| authorships[1].institutions[0].lineage | https://openalex.org/I2799810450 |
| authorships[1].institutions[0].country_code | IL |
| authorships[1].institutions[0].display_name | Sheba Medical Center |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | Lior Frid |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Multiple Sclerosis Center, Sheba Medical Center, Tel-Hashomer, Israel |
| authorships[2].author.id | https://openalex.org/A5051392713 |
| authorships[2].author.orcid | https://orcid.org/0000-0001-7999-0868 |
| authorships[2].author.display_name | Alon Kalron |
| authorships[2].countries | IL |
| authorships[2].affiliations[0].institution_ids | https://openalex.org/I16391192 |
| authorships[2].affiliations[0].raw_affiliation_string | Department of Physical Therapy, School of Health Professions, Faculty of Medical and Health Sciences, Tel-Aviv University, Chaim Levanon st 55, Tel-Aviv, 697881, Israel |
| authorships[2].affiliations[1].institution_ids | https://openalex.org/I16391192 |
| authorships[2].affiliations[1].raw_affiliation_string | Sagol School of Neuroscience, Tel-Aviv University, Tel-Aviv, Israel |
| authorships[2].institutions[0].id | https://openalex.org/I16391192 |
| authorships[2].institutions[0].ror | https://ror.org/04mhzgx49 |
| authorships[2].institutions[0].type | education |
| authorships[2].institutions[0].lineage | https://openalex.org/I16391192 |
| authorships[2].institutions[0].country_code | IL |
| authorships[2].institutions[0].display_name | Tel Aviv University |
| authorships[2].author_position | last |
| authorships[2].raw_author_name | Alon Kalron |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Department of Physical Therapy, School of Health Professions, Faculty of Medical and Health Sciences, Tel-Aviv University, Chaim Levanon st 55, Tel-Aviv, 697881, Israel, Sagol School of Neuroscience, Tel-Aviv University, Tel-Aviv, Israel |
| has_content.pdf | False |
| has_content.grobid_xml | False |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | https://doi.org/10.1177/17562864251321696 |
| open_access.oa_status | gold |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Sustained-release oral dalfampridine appears to have no impact on upper extremity function in people with multiple sclerosis: a randomized controlled trial |
| has_fulltext | False |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10137 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9995999932289124 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2734 |
| primary_topic.subfield.display_name | Pathology and Forensic Medicine |
| primary_topic.display_name | Multiple Sclerosis Research Studies |
| related_works | https://openalex.org/W2340005245, https://openalex.org/W1547508840, https://openalex.org/W2052773812, https://openalex.org/W2428872210, https://openalex.org/W2181360123, https://openalex.org/W3029411384, https://openalex.org/W2136279810, https://openalex.org/W2969572049, https://openalex.org/W2474610971, https://openalex.org/W2381409403 |
| cited_by_count | 0 |
| locations_count | 4 |
| best_oa_location.id | doi:10.1177/17562864251321696 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S7261370 |
| best_oa_location.source.issn | 1756-2856, 1756-2864 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | True |
| best_oa_location.source.issn_l | 1756-2856 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | True |
| best_oa_location.source.display_name | Therapeutic Advances in Neurological Disorders |
| best_oa_location.source.host_organization | https://openalex.org/P4310320017 |
| best_oa_location.source.host_organization_name | SAGE Publishing |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310320017 |
| best_oa_location.source.host_organization_lineage_names | SAGE Publishing |
| best_oa_location.license | |
| best_oa_location.pdf_url | |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | Therapeutic Advances in Neurological Disorders |
| best_oa_location.landing_page_url | https://doi.org/10.1177/17562864251321696 |
| primary_location.id | doi:10.1177/17562864251321696 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S7261370 |
| primary_location.source.issn | 1756-2856, 1756-2864 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | True |
| primary_location.source.issn_l | 1756-2856 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | True |
| primary_location.source.display_name | Therapeutic Advances in Neurological Disorders |
| primary_location.source.host_organization | https://openalex.org/P4310320017 |
| primary_location.source.host_organization_name | SAGE Publishing |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310320017 |
| primary_location.source.host_organization_lineage_names | SAGE Publishing |
| primary_location.license | |
| primary_location.pdf_url | |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | Therapeutic Advances in Neurological Disorders |
| primary_location.landing_page_url | https://doi.org/10.1177/17562864251321696 |
| publication_date | 2025-01-01 |
| publication_year | 2025 |
| referenced_works | https://openalex.org/W2039599652, https://openalex.org/W1752985724, https://openalex.org/W2104262787, https://openalex.org/W2341624928, https://openalex.org/W3129960831, https://openalex.org/W2980477372, https://openalex.org/W2972512991, https://openalex.org/W1980168320, https://openalex.org/W3004245110, https://openalex.org/W2551011356, https://openalex.org/W612998720, https://openalex.org/W2151261385, https://openalex.org/W2329985485, https://openalex.org/W2777074421, https://openalex.org/W2077764376, https://openalex.org/W2142887818, https://openalex.org/W2589005373, https://openalex.org/W1977086599, https://openalex.org/W3038516209, https://openalex.org/W2163908603, https://openalex.org/W1988764089, https://openalex.org/W2070306496, https://openalex.org/W2119292054, https://openalex.org/W2019178494, https://openalex.org/W1999593591, https://openalex.org/W3112416588, https://openalex.org/W2963098570, https://openalex.org/W2520481165, https://openalex.org/W4365478915 |
| referenced_works_count | 29 |
| abstract_inverted_index.1 | 87 |
| abstract_inverted_index.2 | 77, 90, 95 |
| abstract_inverted_index.A | 54 |
| abstract_inverted_index.a | 74 |
| abstract_inverted_index.30 | 61 |
| abstract_inverted_index.In | 59 |
| abstract_inverted_index.No | 148 |
| abstract_inverted_index.To | 39 |
| abstract_inverted_index.at | 84 |
| abstract_inverted_index.in | 6, 25, 28, 51, 172, 194, 218 |
| abstract_inverted_index.is | 4 |
| abstract_inverted_index.mg | 70 |
| abstract_inverted_index.no | 169 |
| abstract_inverted_index.of | 18, 43, 92, 118, 126, 139 |
| abstract_inverted_index.on | 32, 47 |
| abstract_inverted_index.or | 73, 158, 177 |
| abstract_inverted_index.to | 65, 141, 181, 189, 210 |
| abstract_inverted_index.× | 144, 151 |
| abstract_inverted_index.(10 | 69 |
| abstract_inverted_index.Peg | 102 |
| abstract_inverted_index.and | 16, 94, 105, 114, 122 |
| abstract_inverted_index.for | 76, 207 |
| abstract_inverted_index.has | 22 |
| abstract_inverted_index.its | 30, 197 |
| abstract_inverted_index.not | 187 |
| abstract_inverted_index.the | 41, 119, 127, 205 |
| abstract_inverted_index.was | 82 |
| abstract_inverted_index.36). | 132 |
| abstract_inverted_index.Both | 161 |
| abstract_inverted_index.Box, | 104 |
| abstract_inverted_index.Data | 133 |
| abstract_inverted_index.all, | 60 |
| abstract_inverted_index.arm, | 120 |
| abstract_inverted_index.does | 186 |
| abstract_inverted_index.gait | 27 |
| abstract_inverted_index.grip | 110 |
| abstract_inverted_index.limb | 2, 216 |
| abstract_inverted_index.need | 206 |
| abstract_inverted_index.oral | 45 |
| abstract_inverted_index.over | 166 |
| abstract_inverted_index.peak | 108 |
| abstract_inverted_index.pwMS | 62 |
| abstract_inverted_index.this | 219 |
| abstract_inverted_index.time | 145, 152 |
| abstract_inverted_index.were | 63, 134, 154 |
| abstract_inverted_index.with | 8, 168 |
| abstract_inverted_index.Block | 106 |
| abstract_inverted_index.Test, | 103, 107 |
| abstract_inverted_index.These | 202 |
| abstract_inverted_index.Trial | 221 |
| abstract_inverted_index.Upper | 1, 79 |
| abstract_inverted_index.While | 20 |
| abstract_inverted_index.after | 86, 89 |
| abstract_inverted_index.daily | 14 |
| abstract_inverted_index.group | 143, 150 |
| abstract_inverted_index.hand, | 123, 128 |
| abstract_inverted_index.life. | 19 |
| abstract_inverted_index.pwMS, | 29, 195 |
| abstract_inverted_index.pwMS. | 52 |
| abstract_inverted_index.shown | 23 |
| abstract_inverted_index.tests | 100 |
| abstract_inverted_index.time, | 167 |
| abstract_inverted_index.twice | 71 |
| abstract_inverted_index.upper | 33, 48, 173, 191, 215 |
| abstract_inverted_index.using | 98, 136 |
| abstract_inverted_index.week, | 88 |
| abstract_inverted_index.weeks | 91, 96 |
| abstract_inverted_index.Manual | 129 |
| abstract_inverted_index.across | 156 |
| abstract_inverted_index.appear | 188 |
| abstract_inverted_index.beyond | 199 |
| abstract_inverted_index.common | 5 |
| abstract_inverted_index.daily) | 72 |
| abstract_inverted_index.effect | 42 |
| abstract_inverted_index.either | 67 |
| abstract_inverted_index.force, | 111 |
| abstract_inverted_index.groups | 162 |
| abstract_inverted_index.impact | 31 |
| abstract_inverted_index.people | 7 |
| abstract_inverted_index.trends | 165 |
| abstract_inverted_index.trial. | 57 |
| abstract_inverted_index.weeks. | 78 |
| abstract_inverted_index.(9-Hole | 101 |
| abstract_inverted_index.(pwMS), | 11 |
| abstract_inverted_index.2-point | 112 |
| abstract_inverted_index.Ability | 130 |
| abstract_inverted_index.Design: | 53 |
| abstract_inverted_index.ability | 124 |
| abstract_inverted_index.explore | 211 |
| abstract_inverted_index.further | 208 |
| abstract_inverted_index.improve | 190 |
| abstract_inverted_index.measure | 125 |
| abstract_inverted_index.placebo | 75 |
| abstract_inverted_index.quality | 17 |
| abstract_inverted_index.receive | 66 |
| abstract_inverted_index.remains | 36 |
| abstract_inverted_index.similar | 164 |
| abstract_inverted_index.Methods: | 58 |
| abstract_inverted_index.Results: | 147 |
| abstract_inverted_index.analysis | 138 |
| abstract_inverted_index.analyzed | 135 |
| abstract_inverted_index.assessed | 83 |
| abstract_inverted_index.clinical | 99, 157 |
| abstract_inverted_index.efficacy | 24 |
| abstract_inverted_index.evaluate | 40, 142 |
| abstract_inverted_index.findings | 203 |
| abstract_inverted_index.function | 35, 50, 81, 179, 193 |
| abstract_inverted_index.multiple | 9 |
| abstract_inverted_index.observed | 155 |
| abstract_inverted_index.research | 209 |
| abstract_inverted_index.shoulder | 121 |
| abstract_inverted_index.unclear. | 37 |
| abstract_inverted_index.variance | 140 |
| abstract_inverted_index.affecting | 13 |
| abstract_inverted_index.baseline, | 85 |
| abstract_inverted_index.benefits. | 201 |
| abstract_inverted_index.exhibited | 163 |
| abstract_inverted_index.extremity | 34, 49, 80, 174, 192 |
| abstract_inverted_index.improving | 26 |
| abstract_inverted_index.isometric | 109 |
| abstract_inverted_index.outcomes. | 160 |
| abstract_inverted_index.perceived | 178 |
| abstract_inverted_index.sclerosis | 10 |
| abstract_inverted_index.strength, | 176 |
| abstract_inverted_index.targeting | 214 |
| abstract_inverted_index.activities | 15 |
| abstract_inverted_index.dexterity, | 175 |
| abstract_inverted_index.measurable | 170 |
| abstract_inverted_index.randomized | 64 |
| abstract_inverted_index.treatment, | 93 |
| abstract_inverted_index.treatments | 213 |
| abstract_inverted_index.underscore | 204 |
| abstract_inverted_index.Background: | 0 |
| abstract_inverted_index.Conclusion: | 183 |
| abstract_inverted_index.Measurement | 131 |
| abstract_inverted_index.Objectives: | 38 |
| abstract_inverted_index.alternative | 212 |
| abstract_inverted_index.dysfunction | 3, 217 |
| abstract_inverted_index.limitations | 198 |
| abstract_inverted_index.population. | 220 |
| abstract_inverted_index.randomized, | 55 |
| abstract_inverted_index.significant | 149 |
| abstract_inverted_index.NCT02259361. | 224 |
| abstract_inverted_index.attributable | 180 |
| abstract_inverted_index.gait-related | 200 |
| abstract_inverted_index.highlighting | 196 |
| abstract_inverted_index.improvements | 171 |
| abstract_inverted_index.interactions | 153 |
| abstract_inverted_index.(disabilities | 117 |
| abstract_inverted_index.dalfampridine | 21, 46, 68, 185 |
| abstract_inverted_index.interactions. | 146 |
| abstract_inverted_index.registration: | 222 |
| abstract_inverted_index.self-reported | 115, 159 |
| abstract_inverted_index.significantly | 12 |
| abstract_inverted_index.dalfampridine. | 182 |
| abstract_inverted_index.post-treatment | 97 |
| abstract_inverted_index.questionnaires | 116 |
| abstract_inverted_index.discrimination) | 113 |
| abstract_inverted_index.Sustained-release | 184 |
| abstract_inverted_index.repeated-measures | 137 |
| abstract_inverted_index.sustained-release | 44 |
| abstract_inverted_index.ClinicalTrials.gov | 223 |
| abstract_inverted_index.placebo-controlled | 56 |
| cited_by_percentile_year | |
| countries_distinct_count | 1 |
| institutions_distinct_count | 3 |
| sustainable_development_goals[0].id | https://metadata.un.org/sdg/16 |
| sustainable_development_goals[0].score | 0.5899999737739563 |
| sustainable_development_goals[0].display_name | Peace, Justice and strong institutions |
| sustainable_development_goals[1].id | https://metadata.un.org/sdg/10 |
| sustainable_development_goals[1].score | 0.4099999964237213 |
| sustainable_development_goals[1].display_name | Reduced inequalities |
| citation_normalized_percentile.value | 0.07042294 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | False |