Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—SOD and Antimicrobial Activities Article Swipe
 YOU?
·
· 2018
· Open Access
·
· DOI: https://doi.org/10.1155/2018/8759372
YOU?
·
· 2018
· Open Access
·
· DOI: https://doi.org/10.1155/2018/8759372
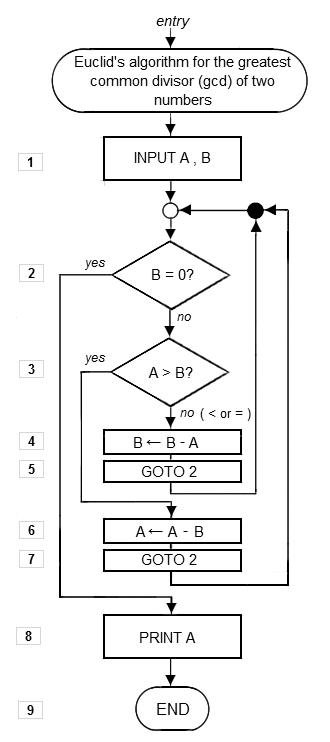
A bidentate (N- and O-) imine-based ligand (L 1 ) and its metal complexes of types [(L 1 ) 2 ] (C 1 ) , [(L 1 )(Phen)] (C 2 ) , [(L 1 ) 2 ] (C 3 ) , and [(L 1 )(Phen)] (C 4 ) (L 1 = 2-((E)-(6-fluorobenzo[d]thiazol-2-ylimino)methyl)-4-chlorophenol and phen = 1,10-phenanthroline) were synthesized as potential chemotherapeutic drug candidates. The prepared complexes were structurally characterized by spectral techniques (NMR, FT-IR, LC-MS, EPR, and electronic absorption), thermogravimetric analysis (TGA/DTA), magnetic moment, and CHNO elemental analysis. Spectroscopic studies suggested the distorted octahedral structure for all complexes. In vitro bioassay studies include binding and nuclease activities of the ligand and its complexes with target calf thymus- (CT-) DNA were carried out by employing UV-Vis, fluorescence spectroscopy, viscosity, and gel electrophoresis techniques. The extent of binding propensity was determined quantitatively by and values which revealed a higher binding affinity for C 2 and C 4 as compared to C 1 and C 3 . In addition, the scavenging superoxide anion free radical () activity of metal complexes was determined by nitroblue tetrazolium (NBT) light reduction assay. Molecular docking studies with DNA and SOD enzyme were also carried out on these compounds. The antimicrobial study has shown that all the compounds are potential antibacterial agents against Gram-negative bacterial strains and better antifungal agents with respect to standard drugs used.
Related Topics
- Type
- article
- Language
- lv
- Landing Page
- https://doi.org/10.1155/2018/8759372
- http://downloads.hindawi.com/archive/2018/8759372.pdf
- OA Status
- hybrid
- Cited By
- 32
- References
- 59
- Related Works
- 10
- OpenAlex ID
- https://openalex.org/W2789798940
Raw OpenAlex JSON
- OpenAlex ID
-
https://openalex.org/W2789798940Canonical identifier for this work in OpenAlex
- DOI
-
https://doi.org/10.1155/2018/8759372Digital Object Identifier
- Title
-
Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—SOD and Antimicrobial ActivitiesWork title
- Type
-
articleOpenAlex work type
- Language
-
lvPrimary language
- Publication year
-
2018Year of publication
- Publication date
-
2018-03-01Full publication date if available
- Authors
-
Savithri Kumar, B.C. Vasantha Kumar, Vivek Hamse Kameshwar, H. D. RevanasiddappaList of authors in order
- Landing page
-
https://doi.org/10.1155/2018/8759372Publisher landing page
- PDF URL
-
https://downloads.hindawi.com/archive/2018/8759372.pdfDirect link to full text PDF
- Open access
-
YesWhether a free full text is available
- OA status
-
hybridOpen access status per OpenAlex
- OA URL
-
https://downloads.hindawi.com/archive/2018/8759372.pdfDirect OA link when available
- Concepts
-
Algorithm, Computer scienceTop concepts (fields/topics) attached by OpenAlex
- Cited by
-
32Total citation count in OpenAlex
- Citations by year (recent)
-
2025: 3, 2024: 2, 2023: 4, 2022: 4, 2021: 8Per-year citation counts (last 5 years)
- References (count)
-
59Number of works referenced by this work
- Related works (count)
-
10Other works algorithmically related by OpenAlex
Full payload
| id | https://openalex.org/W2789798940 |
|---|---|
| doi | https://doi.org/10.1155/2018/8759372 |
| ids.doi | https://doi.org/10.1155/2018/8759372 |
| ids.mag | 2789798940 |
| ids.openalex | https://openalex.org/W2789798940 |
| fwci | 2.11774949 |
| type | article |
| title | Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—SOD and Antimicrobial Activities |
| biblio.issue | |
| biblio.volume | 2018 |
| biblio.last_page | 15 |
| biblio.first_page | 1 |
| topics[0].id | https://openalex.org/T10163 |
| topics[0].field.id | https://openalex.org/fields/27 |
| topics[0].field.display_name | Medicine |
| topics[0].score | 0.9995999932289124 |
| topics[0].domain.id | https://openalex.org/domains/4 |
| topics[0].domain.display_name | Health Sciences |
| topics[0].subfield.id | https://openalex.org/subfields/2730 |
| topics[0].subfield.display_name | Oncology |
| topics[0].display_name | Metal complexes synthesis and properties |
| topics[1].id | https://openalex.org/T13160 |
| topics[1].field.id | https://openalex.org/fields/16 |
| topics[1].field.display_name | Chemistry |
| topics[1].score | 0.9994999766349792 |
| topics[1].domain.id | https://openalex.org/domains/3 |
| topics[1].domain.display_name | Physical Sciences |
| topics[1].subfield.id | https://openalex.org/subfields/1605 |
| topics[1].subfield.display_name | Organic Chemistry |
| topics[1].display_name | Synthesis and Biological Evaluation |
| topics[2].id | https://openalex.org/T14099 |
| topics[2].field.id | https://openalex.org/fields/16 |
| topics[2].field.display_name | Chemistry |
| topics[2].score | 0.9991000294685364 |
| topics[2].domain.id | https://openalex.org/domains/3 |
| topics[2].domain.display_name | Physical Sciences |
| topics[2].subfield.id | https://openalex.org/subfields/1605 |
| topics[2].subfield.display_name | Organic Chemistry |
| topics[2].display_name | Synthesis and Characterization of Heterocyclic Compounds |
| is_xpac | False |
| apc_list | |
| apc_paid | |
| concepts[0].id | https://openalex.org/C11413529 |
| concepts[0].level | 1 |
| concepts[0].score | 0.38007405400276184 |
| concepts[0].wikidata | https://www.wikidata.org/wiki/Q8366 |
| concepts[0].display_name | Algorithm |
| concepts[1].id | https://openalex.org/C41008148 |
| concepts[1].level | 0 |
| concepts[1].score | 0.17916560173034668 |
| concepts[1].wikidata | https://www.wikidata.org/wiki/Q21198 |
| concepts[1].display_name | Computer science |
| keywords[0].id | https://openalex.org/keywords/algorithm |
| keywords[0].score | 0.38007405400276184 |
| keywords[0].display_name | Algorithm |
| keywords[1].id | https://openalex.org/keywords/computer-science |
| keywords[1].score | 0.17916560173034668 |
| keywords[1].display_name | Computer science |
| language | lv |
| locations[0].id | doi:10.1155/2018/8759372 |
| locations[0].is_oa | True |
| locations[0].source.id | https://openalex.org/S100687168 |
| locations[0].source.issn | 1687-9449, 1687-9457 |
| locations[0].source.type | journal |
| locations[0].source.is_oa | False |
| locations[0].source.issn_l | 1687-9449 |
| locations[0].source.is_core | True |
| locations[0].source.is_in_doaj | False |
| locations[0].source.display_name | International Journal of Spectroscopy |
| locations[0].source.host_organization | https://openalex.org/P4310319869 |
| locations[0].source.host_organization_name | Hindawi Publishing Corporation |
| locations[0].source.host_organization_lineage | https://openalex.org/P4310319869 |
| locations[0].license | cc-by |
| locations[0].pdf_url | http://downloads.hindawi.com/archive/2018/8759372.pdf |
| locations[0].version | publishedVersion |
| locations[0].raw_type | journal-article |
| locations[0].license_id | https://openalex.org/licenses/cc-by |
| locations[0].is_accepted | True |
| locations[0].is_published | True |
| locations[0].raw_source_name | International Journal of Spectroscopy |
| locations[0].landing_page_url | https://doi.org/10.1155/2018/8759372 |
| indexed_in | crossref |
| authorships[0].author.id | https://openalex.org/A5019597403 |
| authorships[0].author.orcid | |
| authorships[0].author.display_name | Savithri Kumar |
| authorships[0].countries | IN |
| authorships[0].affiliations[0].institution_ids | https://openalex.org/I204743663 |
| authorships[0].affiliations[0].raw_affiliation_string | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| authorships[0].institutions[0].id | https://openalex.org/I204743663 |
| authorships[0].institutions[0].ror | https://ror.org/012bxv356 |
| authorships[0].institutions[0].type | education |
| authorships[0].institutions[0].lineage | https://openalex.org/I204743663 |
| authorships[0].institutions[0].country_code | IN |
| authorships[0].institutions[0].display_name | University of Mysore |
| authorships[0].author_position | first |
| authorships[0].raw_author_name | K. Savithri |
| authorships[0].is_corresponding | False |
| authorships[0].raw_affiliation_strings | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| authorships[1].author.id | https://openalex.org/A5018770115 |
| authorships[1].author.orcid | |
| authorships[1].author.display_name | B.C. Vasantha Kumar |
| authorships[1].countries | IN |
| authorships[1].affiliations[0].institution_ids | https://openalex.org/I204743663 |
| authorships[1].affiliations[0].raw_affiliation_string | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| authorships[1].institutions[0].id | https://openalex.org/I204743663 |
| authorships[1].institutions[0].ror | https://ror.org/012bxv356 |
| authorships[1].institutions[0].type | education |
| authorships[1].institutions[0].lineage | https://openalex.org/I204743663 |
| authorships[1].institutions[0].country_code | IN |
| authorships[1].institutions[0].display_name | University of Mysore |
| authorships[1].author_position | middle |
| authorships[1].raw_author_name | B. C. Vasantha Kumar |
| authorships[1].is_corresponding | False |
| authorships[1].raw_affiliation_strings | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| authorships[2].author.id | https://openalex.org/A5079821687 |
| authorships[2].author.orcid | https://orcid.org/0000-0002-5808-5583 |
| authorships[2].author.display_name | Vivek Hamse Kameshwar |
| authorships[2].affiliations[0].raw_affiliation_string | Sri Ram Chem, R&D Center, JCK Industrial Park, Belagola Industrial Area, Mysuru 571 606, India |
| authorships[2].author_position | middle |
| authorships[2].raw_author_name | H. K. Vivek |
| authorships[2].is_corresponding | False |
| authorships[2].raw_affiliation_strings | Sri Ram Chem, R&D Center, JCK Industrial Park, Belagola Industrial Area, Mysuru 571 606, India |
| authorships[3].author.id | https://openalex.org/A5025837506 |
| authorships[3].author.orcid | https://orcid.org/0000-0003-0127-3599 |
| authorships[3].author.display_name | H. D. Revanasiddappa |
| authorships[3].countries | IN |
| authorships[3].affiliations[0].institution_ids | https://openalex.org/I204743663 |
| authorships[3].affiliations[0].raw_affiliation_string | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| authorships[3].institutions[0].id | https://openalex.org/I204743663 |
| authorships[3].institutions[0].ror | https://ror.org/012bxv356 |
| authorships[3].institutions[0].type | education |
| authorships[3].institutions[0].lineage | https://openalex.org/I204743663 |
| authorships[3].institutions[0].country_code | IN |
| authorships[3].institutions[0].display_name | University of Mysore |
| authorships[3].author_position | last |
| authorships[3].raw_author_name | H. D. Revanasiddappa |
| authorships[3].is_corresponding | True |
| authorships[3].raw_affiliation_strings | Department of Studies in Chemistry, University of Mysore, Mysuru, Karnataka 570 006, India |
| has_content.pdf | True |
| has_content.grobid_xml | True |
| is_paratext | False |
| open_access.is_oa | True |
| open_access.oa_url | http://downloads.hindawi.com/archive/2018/8759372.pdf |
| open_access.oa_status | hybrid |
| open_access.any_repository_has_fulltext | False |
| created_date | 2025-10-10T00:00:00 |
| display_name | Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—SOD and Antimicrobial Activities |
| has_fulltext | True |
| is_retracted | False |
| updated_date | 2025-11-06T03:46:38.306776 |
| primary_topic.id | https://openalex.org/T10163 |
| primary_topic.field.id | https://openalex.org/fields/27 |
| primary_topic.field.display_name | Medicine |
| primary_topic.score | 0.9995999932289124 |
| primary_topic.domain.id | https://openalex.org/domains/4 |
| primary_topic.domain.display_name | Health Sciences |
| primary_topic.subfield.id | https://openalex.org/subfields/2730 |
| primary_topic.subfield.display_name | Oncology |
| primary_topic.display_name | Metal complexes synthesis and properties |
| related_works | https://openalex.org/W2051487156, https://openalex.org/W2073681303, https://openalex.org/W2053286651, https://openalex.org/W2181743346, https://openalex.org/W2187401768, https://openalex.org/W2181413294, https://openalex.org/W2052122378, https://openalex.org/W2544423928, https://openalex.org/W2062023542, https://openalex.org/W4313731559 |
| cited_by_count | 32 |
| counts_by_year[0].year | 2025 |
| counts_by_year[0].cited_by_count | 3 |
| counts_by_year[1].year | 2024 |
| counts_by_year[1].cited_by_count | 2 |
| counts_by_year[2].year | 2023 |
| counts_by_year[2].cited_by_count | 4 |
| counts_by_year[3].year | 2022 |
| counts_by_year[3].cited_by_count | 4 |
| counts_by_year[4].year | 2021 |
| counts_by_year[4].cited_by_count | 8 |
| counts_by_year[5].year | 2020 |
| counts_by_year[5].cited_by_count | 7 |
| counts_by_year[6].year | 2019 |
| counts_by_year[6].cited_by_count | 2 |
| counts_by_year[7].year | 2018 |
| counts_by_year[7].cited_by_count | 2 |
| locations_count | 1 |
| best_oa_location.id | doi:10.1155/2018/8759372 |
| best_oa_location.is_oa | True |
| best_oa_location.source.id | https://openalex.org/S100687168 |
| best_oa_location.source.issn | 1687-9449, 1687-9457 |
| best_oa_location.source.type | journal |
| best_oa_location.source.is_oa | False |
| best_oa_location.source.issn_l | 1687-9449 |
| best_oa_location.source.is_core | True |
| best_oa_location.source.is_in_doaj | False |
| best_oa_location.source.display_name | International Journal of Spectroscopy |
| best_oa_location.source.host_organization | https://openalex.org/P4310319869 |
| best_oa_location.source.host_organization_name | Hindawi Publishing Corporation |
| best_oa_location.source.host_organization_lineage | https://openalex.org/P4310319869 |
| best_oa_location.license | cc-by |
| best_oa_location.pdf_url | http://downloads.hindawi.com/archive/2018/8759372.pdf |
| best_oa_location.version | publishedVersion |
| best_oa_location.raw_type | journal-article |
| best_oa_location.license_id | https://openalex.org/licenses/cc-by |
| best_oa_location.is_accepted | True |
| best_oa_location.is_published | True |
| best_oa_location.raw_source_name | International Journal of Spectroscopy |
| best_oa_location.landing_page_url | https://doi.org/10.1155/2018/8759372 |
| primary_location.id | doi:10.1155/2018/8759372 |
| primary_location.is_oa | True |
| primary_location.source.id | https://openalex.org/S100687168 |
| primary_location.source.issn | 1687-9449, 1687-9457 |
| primary_location.source.type | journal |
| primary_location.source.is_oa | False |
| primary_location.source.issn_l | 1687-9449 |
| primary_location.source.is_core | True |
| primary_location.source.is_in_doaj | False |
| primary_location.source.display_name | International Journal of Spectroscopy |
| primary_location.source.host_organization | https://openalex.org/P4310319869 |
| primary_location.source.host_organization_name | Hindawi Publishing Corporation |
| primary_location.source.host_organization_lineage | https://openalex.org/P4310319869 |
| primary_location.license | cc-by |
| primary_location.pdf_url | http://downloads.hindawi.com/archive/2018/8759372.pdf |
| primary_location.version | publishedVersion |
| primary_location.raw_type | journal-article |
| primary_location.license_id | https://openalex.org/licenses/cc-by |
| primary_location.is_accepted | True |
| primary_location.is_published | True |
| primary_location.raw_source_name | International Journal of Spectroscopy |
| primary_location.landing_page_url | https://doi.org/10.1155/2018/8759372 |
| publication_date | 2018-03-01 |
| publication_year | 2018 |
| referenced_works | https://openalex.org/W2487383787, https://openalex.org/W2067316000, https://openalex.org/W832317166, https://openalex.org/W1968724757, https://openalex.org/W2606691120, https://openalex.org/W2081864434, https://openalex.org/W2234534890, https://openalex.org/W2088791119, https://openalex.org/W1977967213, https://openalex.org/W1963844096, https://openalex.org/W2352934708, https://openalex.org/W2468824401, https://openalex.org/W2003018020, https://openalex.org/W2597764601, https://openalex.org/W2187694879, https://openalex.org/W1988457860, https://openalex.org/W2506683632, https://openalex.org/W1974649707, https://openalex.org/W2468149945, https://openalex.org/W2137996531, https://openalex.org/W2083686365, https://openalex.org/W2076153333, https://openalex.org/W2531114072, https://openalex.org/W2051300175, https://openalex.org/W2075578794, https://openalex.org/W2043281920, https://openalex.org/W2077522026, https://openalex.org/W2179425537, https://openalex.org/W2605603056, https://openalex.org/W2217171842, https://openalex.org/W2063136195, https://openalex.org/W2044851985, https://openalex.org/W2319699375, https://openalex.org/W2093220503, https://openalex.org/W2535571677, https://openalex.org/W2410671543, https://openalex.org/W1979973356, https://openalex.org/W1991276559, https://openalex.org/W2591916049, https://openalex.org/W4241697999, https://openalex.org/W1978472177, https://openalex.org/W1990882038, https://openalex.org/W2041589402, https://openalex.org/W2134559912, https://openalex.org/W2533332371, https://openalex.org/W1539378107, https://openalex.org/W2033532170, https://openalex.org/W2023709279, https://openalex.org/W2430698557, https://openalex.org/W2055758448, https://openalex.org/W2522570141, https://openalex.org/W2061417686, https://openalex.org/W1522997704, https://openalex.org/W2165175723, https://openalex.org/W2142913786, https://openalex.org/W2217700566, https://openalex.org/W2606422863, https://openalex.org/W2314730505, https://openalex.org/W1966284131 |
| referenced_works_count | 59 |
| abstract_inverted_index.) | 9, 24, 29, 42, 53, 58, 73 |
| abstract_inverted_index., | 30, 43, 59 |
| abstract_inverted_index.. | 197 |
| abstract_inverted_index.1 | 8, 23, 28, 38, 52, 69, 75, 193 |
| abstract_inverted_index.2 | 25, 41, 54, 185 |
| abstract_inverted_index.3 | 57, 196 |
| abstract_inverted_index.4 | 72, 188 |
| abstract_inverted_index.= | 76, 80 |
| abstract_inverted_index.A | 0 |
| abstract_inverted_index.C | 184, 187, 192, 195 |
| abstract_inverted_index.] | 26, 55 |
| abstract_inverted_index.a | 179 |
| abstract_inverted_index.(C | 27, 40, 56, 71 |
| abstract_inverted_index.(L | 7, 74 |
| abstract_inverted_index.In | 124, 198 |
| abstract_inverted_index.as | 84, 189 |
| abstract_inverted_index.by | 95, 148, 166, 217 |
| abstract_inverted_index.of | 14, 133, 160, 212 |
| abstract_inverted_index.on | 236 |
| abstract_inverted_index.to | 191, 262 |
| abstract_inverted_index.(N- | 2 |
| abstract_inverted_index.DNA | 144, 228 |
| abstract_inverted_index.O-) | 4 |
| abstract_inverted_index.SOD | 230 |
| abstract_inverted_index.The | 89, 158, 239 |
| abstract_inverted_index.all | 122, 245 |
| abstract_inverted_index.and | 3, 10, 60, 78, 102, 110, 130, 136, 154, 170, 186, 194, 229, 256 |
| abstract_inverted_index.are | 248 |
| abstract_inverted_index.for | 121, 183 |
| abstract_inverted_index.gel | 155 |
| abstract_inverted_index.has | 242 |
| abstract_inverted_index.its | 11, 137 |
| abstract_inverted_index.out | 147, 235 |
| abstract_inverted_index.the | 117, 134, 200, 246 |
| abstract_inverted_index.was | 163, 215 |
| abstract_inverted_index.CHNO | 111 |
| abstract_inverted_index.EPR, | 101 |
| abstract_inverted_index.also | 233 |
| abstract_inverted_index.calf | 141 |
| abstract_inverted_index.drug | 87 |
| abstract_inverted_index.free | 204 |
| abstract_inverted_index.phen | 79 |
| abstract_inverted_index.that | 244 |
| abstract_inverted_index.were | 82, 92, 145, 232 |
| abstract_inverted_index.with | 139, 227, 260 |
| abstract_inverted_index.(CT-) | 143 |
| abstract_inverted_index.(NBT) | 220 |
| abstract_inverted_index.(NMR, | 98 |
| abstract_inverted_index.anion | 203 |
| abstract_inverted_index.drugs | 264 |
| abstract_inverted_index.light | 221 |
| abstract_inverted_index.metal | 12, 213 |
| abstract_inverted_index.shown | 243 |
| abstract_inverted_index.study | 241 |
| abstract_inverted_index.these | 237 |
| abstract_inverted_index.types | 15 |
| abstract_inverted_index.used. | 265 |
| abstract_inverted_index.vitro | 125 |
| abstract_inverted_index.which | 177 |
| abstract_inverted_index.FT-IR, | 99 |
| abstract_inverted_index.LC-MS, | 100 |
| abstract_inverted_index.agents | 251, 259 |
| abstract_inverted_index.assay. | 223 |
| abstract_inverted_index.better | 257 |
| abstract_inverted_index.enzyme | 231 |
| abstract_inverted_index.extent | 159 |
| abstract_inverted_index.higher | 180 |
| abstract_inverted_index.ligand | 6, 135 |
| abstract_inverted_index.target | 140 |
| abstract_inverted_index.values | 176 |
| abstract_inverted_index.UV-Vis, | 150 |
| abstract_inverted_index.against | 252 |
| abstract_inverted_index.binding | 129, 161, 181 |
| abstract_inverted_index.carried | 146, 234 |
| abstract_inverted_index.docking | 225 |
| abstract_inverted_index.include | 128 |
| abstract_inverted_index.moment, | 109 |
| abstract_inverted_index.radical | 205 |
| abstract_inverted_index.respect | 261 |
| abstract_inverted_index.strains | 255 |
| abstract_inverted_index.studies | 115, 127, 226 |
| abstract_inverted_index.thymus- | 142 |
| abstract_inverted_index.)(Phen)] | 39, 70 |
| abstract_inverted_index.activity | 211 |
| abstract_inverted_index.affinity | 182 |
| abstract_inverted_index.analysis | 106 |
| abstract_inverted_index.bioassay | 126 |
| abstract_inverted_index.compared | 190 |
| abstract_inverted_index.magnetic | 108 |
| abstract_inverted_index.nuclease | 131 |
| abstract_inverted_index.prepared | 90 |
| abstract_inverted_index.revealed | 178 |
| abstract_inverted_index.spectral | 96 |
| abstract_inverted_index.standard | 263 |
| abstract_inverted_index.<mml:math | 167, 171 |
| abstract_inverted_index.Molecular | 224 |
| abstract_inverted_index.addition, | 199 |
| abstract_inverted_index.analysis. | 113 |
| abstract_inverted_index.bacterial | 254 |
| abstract_inverted_index.bidentate | 1 |
| abstract_inverted_index.complexes | 13, 91, 138, 214 |
| abstract_inverted_index.compounds | 247 |
| abstract_inverted_index.distorted | 118 |
| abstract_inverted_index.elemental | 112 |
| abstract_inverted_index.employing | 149 |
| abstract_inverted_index.nitroblue | 218 |
| abstract_inverted_index.potential | 85, 249 |
| abstract_inverted_index.reduction | 222 |
| abstract_inverted_index.structure | 120 |
| abstract_inverted_index.suggested | 116 |
| abstract_inverted_index.(<mml:math | 206 |
| abstract_inverted_index.(TGA/DTA), | 107 |
| abstract_inverted_index.[<mml:math | 16, 31, 44, 61 |
| abstract_inverted_index.activities | 132 |
| abstract_inverted_index.antifungal | 258 |
| abstract_inverted_index.complexes. | 123 |
| abstract_inverted_index.compounds. | 238 |
| abstract_inverted_index.determined | 164, 216 |
| abstract_inverted_index.electronic | 103 |
| abstract_inverted_index.octahedral | 119 |
| abstract_inverted_index.propensity | 162 |
| abstract_inverted_index.scavenging | 201 |
| abstract_inverted_index.superoxide | 202 |
| abstract_inverted_index.techniques | 97 |
| abstract_inverted_index.viscosity, | 153 |
| abstract_inverted_index.candidates. | 88 |
| abstract_inverted_index.imine-based | 5 |
| abstract_inverted_index.synthesized | 83 |
| abstract_inverted_index.techniques. | 157 |
| abstract_inverted_index.tetrazolium | 219 |
| abstract_inverted_index.absorption), | 104 |
| abstract_inverted_index.fluorescence | 151 |
| abstract_inverted_index.structurally | 93 |
| abstract_inverted_index.Gram-negative | 253 |
| abstract_inverted_index.Spectroscopic | 114 |
| abstract_inverted_index.antibacterial | 250 |
| abstract_inverted_index.antimicrobial | 240 |
| abstract_inverted_index.characterized | 94 |
| abstract_inverted_index.spectroscopy, | 152 |
| abstract_inverted_index.quantitatively | 165 |
| abstract_inverted_index.electrophoresis | 156 |
| abstract_inverted_index.chemotherapeutic | 86 |
| abstract_inverted_index.thermogravimetric | 105 |
| abstract_inverted_index.1,10-phenanthroline) | 81 |
| abstract_inverted_index.mathvariant="normal">C</mml:mi><mml:mi | 19, 34, 47, 64 |
| abstract_inverted_index.mathvariant="normal">I</mml:mi><mml:mi | 21, 36, 49, 50, 66, 67 |
| abstract_inverted_index.mathvariant="normal">s</mml:mi><mml:mi | 174 |
| abstract_inverted_index.id="M1"><mml:mrow><mml:msup><mml:mrow><mml:mi | 18 |
| abstract_inverted_index.id="M2"><mml:mrow><mml:msup><mml:mrow><mml:mi | 33 |
| abstract_inverted_index.id="M3"><mml:mrow><mml:msup><mml:mrow><mml:mi | 46 |
| abstract_inverted_index.id="M4"><mml:mrow><mml:msup><mml:mrow><mml:mi | 63 |
| abstract_inverted_index.id="M7"><mml:mrow><mml:msup><mml:mrow><mml:mi | 208 |
| abstract_inverted_index.xmlns:mml="http://www.w3.org/1998/Math/MathML" | 17, 32, 45, 62, 168, 172, 207 |
| abstract_inverted_index.mathvariant="normal">o</mml:mi></mml:mrow><mml:mrow><mml:mi | 48, 65 |
| abstract_inverted_index.mathvariant="normal">u</mml:mi></mml:mrow><mml:mrow><mml:mi | 20, 35 |
| abstract_inverted_index.2-((E)-(6-fluorobenzo[d]thiazol-2-ylimino)methyl)-4-chlorophenol | 77 |
| abstract_inverted_index.mathvariant="normal">v</mml:mi></mml:mrow></mml:msub></mml:mrow></mml:math> | 175 |
| abstract_inverted_index.mathvariant="normal">2</mml:mn></mml:mrow></mml:msup></mml:mrow></mml:math>) | 210 |
| abstract_inverted_index.mathvariant="normal">I</mml:mi></mml:mrow></mml:msup></mml:mrow></mml:math>(L | 22, 37, 51, 68 |
| abstract_inverted_index.id="M6"><mml:mrow><mml:msub><mml:mrow><mml:mi>K</mml:mi></mml:mrow><mml:mrow><mml:mi | 173 |
| abstract_inverted_index.mathvariant="normal">O</mml:mi></mml:mrow><mml:mrow><mml:mo>∙</mml:mo><mml:mo>-</mml:mo><mml:mn | 209 |
| abstract_inverted_index.id="M5"><mml:mrow><mml:msub><mml:mrow><mml:mi>K</mml:mi></mml:mrow><mml:mrow><mml:mi>b</mml:mi></mml:mrow></mml:msub></mml:mrow></mml:math> | 169 |
| cited_by_percentile_year.max | 99 |
| cited_by_percentile_year.min | 94 |
| corresponding_author_ids | https://openalex.org/A5025837506 |
| countries_distinct_count | 1 |
| institutions_distinct_count | 4 |
| corresponding_institution_ids | https://openalex.org/I204743663 |
| citation_normalized_percentile.value | 0.88120099 |
| citation_normalized_percentile.is_in_top_1_percent | False |
| citation_normalized_percentile.is_in_top_10_percent | True |