Azimuthal Quantum Number
Swipe Azimuthal Quantum Number Vs...
Azimuthal Quantum Number
Swipe Azimuthal Quantum Number Vs...

Explanipedia Public Learning Modules Vs Mis Dis Mal Information:
Azimuthal Quantum Number News
Loading news…
Description
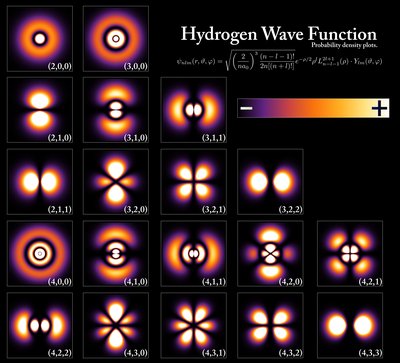
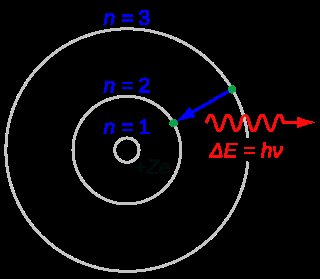
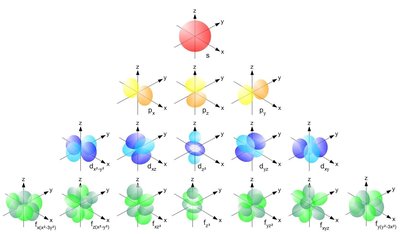
In quantum mechanics, the azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number n, the magnetic quantum number mℓ, and the spin quantum number ms). It is also known as the orbital angular momentum quantum number , orbital quantum number, subsidiary quantum number, or second quantum number , and is symbolized as ℓ (pronounced ell ).
Related
MoreTags
Courage
(28.3K)
Reliability
(25.8K)
Determination
(24.9K)
Authenticity
(22.8K)
Perseverance
(20.3K)
Integrity
(18.7K)
Flexibility
(16.1K)
Respect
(12.3K)
SelfDiscipline
(10.8K)
Tenacious
(9,258)
Tolerance
(8,764)
Progress
(3,270)
Simplicity
(2,763)
Energy
(2,586)
Optimistic
(1,839)
Physics
(1,351)
Equations
(387)
Quantum
(245)
QuantumMechanics
(173)
Principles
(87)
AtomicStructure
(34)
QuantumTheory
(23)
WaveFunction
(8)
Orbitals
(8)
QuantumNumbers
(2)
ElectronOrbitals
(1)
Collections
No collections available for this topic.
Details
- Slug: azimuthal-quantum-number
- Added: Jul 20, 2024