Description
Boyle's law , also referred to as the Boyle–Mariotte law , or, Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as:
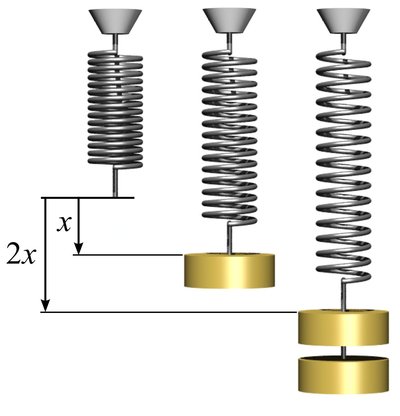
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
Mathematically, Boyle's law can be stated as:
or
where P is the pressure of the gas, V is the volume of the gas, and k is a constant.
Boyle's law states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. When comparing the same substance under two different sets of conditions, the law can be expressed as:
P 1 V 1 = P 2 V 2 . {\displaystyle P_{1}V_{1}=P_{2}V_{2}.}
showing that as volume increases, the pressure of a gas decreases proportionally, and vice versa. Boyle's law is named after Robert Boyle, who published the original law in 1662.