Description
Cabozantinib , sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.
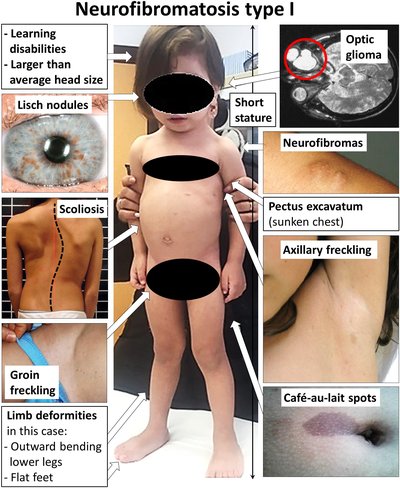
In November 2012, cabozantinib in its capsule formulation was approved by the US Food and Drug Administration (FDA) under the name Cometriq for treating people with medullary thyroid cancer. The capsule form was approved in the European Union for the same purpose in 2014. In April 2016, the FDA granted approval for marketing the tablet formulation (Cabometyx) as a second line treatment for kidney cancer and the same was approved in the European Union in September of that year. The brands Cometriq and Cabometyx have different formulations and are not interchangeable.