Description
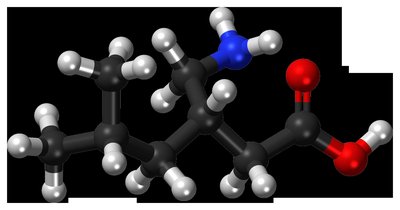
Ketorolac , sold under the brand names Toradol , and Biorolac among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain. Specifically it is recommended for moderate to severe pain. Recommended duration of treatment is less than six days, and in Switzerland not more than two days. It is used by mouth, by nose, by injection into a vein or muscle, and as eye drops. Effects begin within an hour and last for up to eight hours.
Common side effects include sleepiness, dizziness, abdominal pain, swelling, and nausea. Serious side effects may include stomach bleeding, kidney failure, heart attacks, bronchospasm, heart failure, and anaphylaxis. Use is not recommended during the last part of pregnancy or during breastfeeding. Ketorolac works by blocking cyclooxygenase 1 and 2 (COX1 and COX2), thereby decreasing production of prostaglandins.
Ketorolac was patented in 1976 and approved for medical use in 1989. It is available as a generic medication. In 2020, it was the 249th most commonly prescribed medication in the United States, with more than 1 million prescriptions.
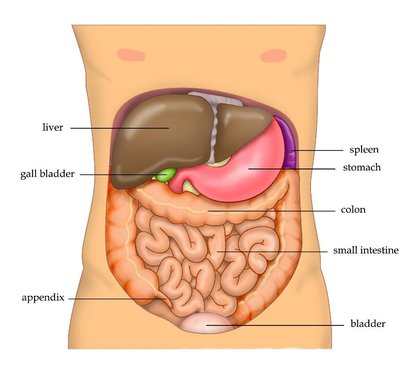
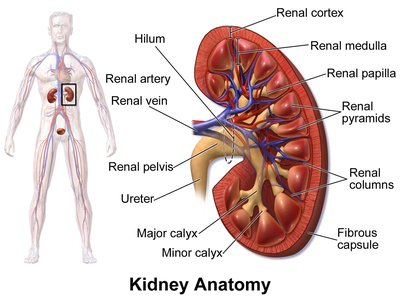
Due to a series of deaths due to gastrointestinal bleeding and kidney failure, ketorolac as a pain medication was removed from the German market in 1993. When ketorolac was introduced into Germany, it was often mis-used as an opioid replacement in pain therapy because its side effects were perceived as much less severe, it did not produce any dependence, and a dose was effective for 7-8 hours compared to morphine with 3-4 hours. As a very potent prostaglandin inhibitor, ketorolac diminishes the kidney's own defenses against vasoconstriction-related effects, e.g. during blood loss or high endogenous catecholamine levels.