Description
Lamotrigine , sold under the brand name Lamictal among others, is a medication used to treat epilepsy and stabilize mood in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome. In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression; but for patients with bipolar disorder who are not currently symptomatic, it appears to be effective in reducing the risk of future episodes of depression.
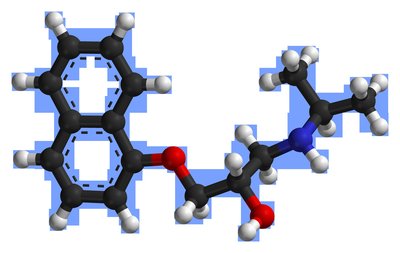
Common side effects include nausea, sleepiness, headache, vomiting, trouble with coordination, and rash. Serious side effects include excessive breakdown of red blood cells, increased risk of suicide, severe skin reaction (Stevens–Johnson syndrome), and allergic reactions, which can be fatal. Lamotrigine is a phenyltriazine, making it chemically different from other anticonvulsants. Its mechanism of action is not clear, but it appears to inhibit release of excitatory neurotransmitters via voltage-sensitive sodium channels and voltage-gated calcium channels in neurons.
Several studies that followed children exposed to ASMs during pregnancy showed that lamotrigine carried a low risk of adverse neurodevelopmental outcomes (cognitive and behavioral) in children when compared to children born to mothers without epilepsy and children born to mothers taking other anti- seizure medications. Data from several pregnancy registries showed that children exposed to lamotrigine during pregnancy had the lowest risk of developing major congenital malformations compared to those exposed to other anti-seizure medications. Risk of major congenital malformations for children exposed to levetiracetam were within the range for children who were not exposed to any ASMs during pregnancy.
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study showed that most blood concentrations in breastfed infants of mothers taking lamotrigine were quite low, especially in relationship to the mother's level and what the fetal level would have been during pregnancy. Median levetiracetam levels in breastfed infants were 28.9% of maternal levels. The Norwegian Mother and Child Cohort Study (MoBa) showed that infant exposure to lamotrigine via breastmilk was not associated with negative neurodevelopment (such as lower IQ and autism spectrum disorder) at 36 months.
Lamotrigine is difficult to titrate once someone is pregnant, given increased metabolism in pregnancy, and should not be started during pregnancy. Additionally, adding lamotrigine to valproic acid during a cross-over carries a high risk of rash.
Lamotrigine is known to undergo significant changes in clearance during pregnancy, so it's important for healthcare providers to frequently check and adjust the lamotrigine doses of pregnant people with epilepsy.
Lamotrigine was first marketed in Ireland in 1991, and approved for use in the United States in 1994. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 62nd most commonly prescribed medication in the United States, with more than 10 million prescriptions.