Description
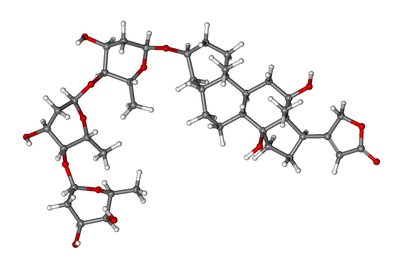
Levetiracetam , sold under the brand name Keppra among others, is a medication used to treat epilepsy. It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.
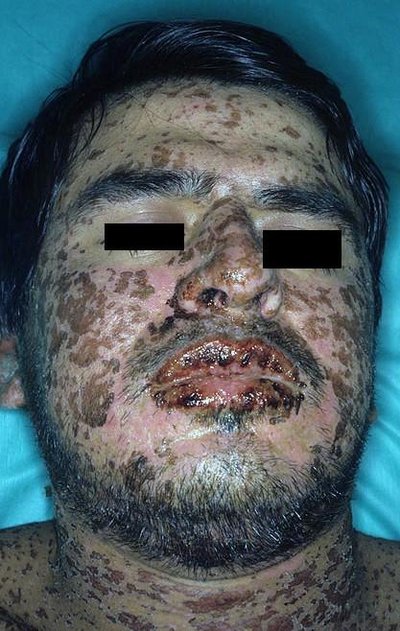
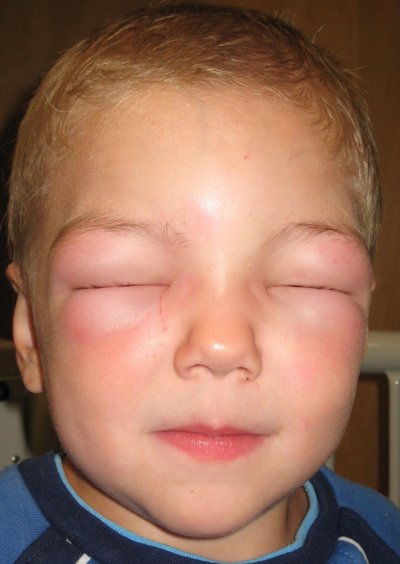
Common side effects of levetiracetam include sleepiness, dizziness, feeling tired, and aggression. Severe side effects may include psychosis, suicide, and allergic reactions such as Stevens–Johnson syndrome or anaphylaxis. Levetiracetam is the S-enantiomer of etiracetam. Its mechanism of action is not yet clear.
Several studies that followed children exposed to anti-seizure medications (ASMs) during pregnancy showed that levetiracetam carried a low risk of adverse neurodevelopmental outcomes (cognitive and behavioral) in children when compared to children born to mothers without epilepsy and children born to mothers taking other anti-seizure medications. Data from several pregnancy registries showed that children exposed to levetiracetam during pregnancy had the lowest risk of developing major congenital malformations compared to those exposed to other anti-seizure medications. Risk of major congenital malformations for children exposed to levetiracetam were within the range for children who were not exposed to any ASMs during pregnancy.
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study showed that most blood concentrations in breastfed infants of mothers taking levetiracetam were quite low, especially in relationship to the mother's level and what the fetal level would have been during pregnancy. Median levetiracetam levels in breastfed infants were 5.3% of maternal levels. The Norwegian Mother and Child Cohort Study (MoBa) showed that infant exposure to levetiracetam via breastmilk was not associated with negative neurodevelopment (such as lower IQ and autism spectrum disorder) at 36 months.
Levetiracetam does not affect hormonal birth control.
Levetiracetam was approved for medical use in the United States in 1999 and is available as a generic medication. In 2020, it was the 92nd most commonly prescribed medication in the United States, with more than 7 million prescriptions. It is on the World Health Organization's List of Essential Medicines.