Description
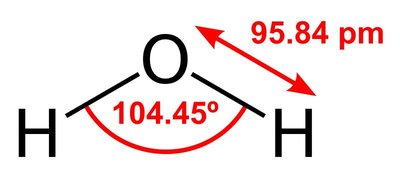
Water activity ( _ a w) is the partial vapor pressure of water in a solution divided by the standard state partial vapor pressure of water. In the field of food science, the standard state is most often defined as pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. Water activity is the thermodynamic activity of water as solvent and the relative humidity of the surrounding air after equilibration. As temperature increases, _a w typically increases, except in some products with crystalline salt or sugar.
Water migrates from areas of high a w to areas of low a w. For example, if honey ( a w ≈ 0.6) is exposed to humid air ( a w ≈ 0.7), the honey absorbs water from the air. If salami ( a w ≈ 0.87) is exposed to dry air ( a w ≈ 0.5), the salami dries out, which could preserve it or spoil it. Lower a w substances tend to support fewer microorganisms since these get desiccated by the water migration.
Water Activity News
Tags
Collections
No collections available for this topic.